[English] 日本語
 Yorodumi
Yorodumi- EMDB-2685: Density map of GluK2 desensitized state in complex with 2S,4R-4-m... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2685 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Density map of GluK2 desensitized state in complex with 2S,4R-4-methylglutamate | |||||||||
 Map data Map data | Reconstruction of GluK2 desensitized state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Membrane Protein / Membrane Protein /  Ion Channel / Ion Channel /  Glutamate Receptor Glutamate Receptor | |||||||||
| Function / homology |  Function and homology information Function and homology information mossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / negative regulation of synaptic transmission, glutamatergic / regulation of short-term neuronal synaptic plasticity / mossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / negative regulation of synaptic transmission, glutamatergic / regulation of short-term neuronal synaptic plasticity /  inhibitory postsynaptic potential / inhibitory postsynaptic potential /  glutamate receptor activity / ubiquitin conjugating enzyme binding ... glutamate receptor activity / ubiquitin conjugating enzyme binding ... mossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / negative regulation of synaptic transmission, glutamatergic / regulation of short-term neuronal synaptic plasticity / mossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / negative regulation of synaptic transmission, glutamatergic / regulation of short-term neuronal synaptic plasticity /  inhibitory postsynaptic potential / inhibitory postsynaptic potential /  glutamate receptor activity / ubiquitin conjugating enzyme binding / receptor clustering / modulation of excitatory postsynaptic potential / neuronal action potential / regulation of JNK cascade / kainate selective glutamate receptor activity / glutamate receptor activity / ubiquitin conjugating enzyme binding / receptor clustering / modulation of excitatory postsynaptic potential / neuronal action potential / regulation of JNK cascade / kainate selective glutamate receptor activity /  ionotropic glutamate receptor complex / extracellularly glutamate-gated ion channel activity / behavioral fear response / positive regulation of synaptic transmission / glutamate-gated receptor activity / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / ionotropic glutamate receptor complex / extracellularly glutamate-gated ion channel activity / behavioral fear response / positive regulation of synaptic transmission / glutamate-gated receptor activity / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential /  excitatory postsynaptic potential / hippocampal mossy fiber to CA3 synapse / presynaptic modulation of chemical synaptic transmission / excitatory postsynaptic potential / hippocampal mossy fiber to CA3 synapse / presynaptic modulation of chemical synaptic transmission /  regulation of membrane potential / dendrite cytoplasm / regulation of membrane potential / dendrite cytoplasm /  SNARE binding / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / SNARE binding / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential /  synaptic transmission, glutamatergic / synaptic transmission, glutamatergic /  PDZ domain binding / postsynaptic density membrane / regulation of long-term neuronal synaptic plasticity / modulation of chemical synaptic transmission / PDZ domain binding / postsynaptic density membrane / regulation of long-term neuronal synaptic plasticity / modulation of chemical synaptic transmission /  terminal bouton / intracellular calcium ion homeostasis / positive regulation of neuron apoptotic process / terminal bouton / intracellular calcium ion homeostasis / positive regulation of neuron apoptotic process /  presynaptic membrane / chemical synaptic transmission / presynaptic membrane / chemical synaptic transmission /  perikaryon / perikaryon /  postsynaptic membrane / postsynaptic membrane /  scaffold protein binding / neuron apoptotic process / negative regulation of neuron apoptotic process / scaffold protein binding / neuron apoptotic process / negative regulation of neuron apoptotic process /  postsynaptic density / postsynaptic density /  axon / axon /  dendrite / neuronal cell body / glutamatergic synapse / dendrite / neuronal cell body / glutamatergic synapse /  synapse / synapse /  ubiquitin protein ligase binding / ubiquitin protein ligase binding /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Rattus norvegicus (Norway rat) / synthetic construct (others) Rattus norvegicus (Norway rat) / synthetic construct (others) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 7.6 Å cryo EM / Resolution: 7.6 Å | |||||||||
 Authors Authors | Meyerson JR / Kumar J / Chittori S / Rao P / Pierson J / Bartesaghi A / Mayer ML / Subramaniam S | |||||||||
 Citation Citation |  Journal: Nature / Year: 2014 Journal: Nature / Year: 2014Title: Structural mechanism of glutamate receptor activation and desensitization. Authors: Joel R Meyerson / Janesh Kumar / Sagar Chittori / Prashant Rao / Jason Pierson / Alberto Bartesaghi / Mark L Mayer / Sriram Subramaniam /  Abstract: Ionotropic glutamate receptors are ligand-gated ion channels that mediate excitatory synaptic transmission in the vertebrate brain. To gain a better understanding of how structural changes gate ion ...Ionotropic glutamate receptors are ligand-gated ion channels that mediate excitatory synaptic transmission in the vertebrate brain. To gain a better understanding of how structural changes gate ion flux across the membrane, we trapped rat AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and kainate receptor subtypes in their major functional states and analysed the resulting structures using cryo-electron microscopy. We show that transition to the active state involves a 'corkscrew' motion of the receptor assembly, driven by closure of the ligand-binding domain. Desensitization is accompanied by disruption of the amino-terminal domain tetramer in AMPA, but not kainate, receptors with a two-fold to four-fold symmetry transition in the ligand-binding domains in both subtypes. The 7.6 Å structure of a desensitized kainate receptor shows how these changes accommodate channel closing. These findings integrate previous physiological, biochemical and structural analyses of glutamate receptors and provide a molecular explanation for key steps in receptor gating. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2685.map.gz emd_2685.map.gz | 3.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2685-v30.xml emd-2685-v30.xml emd-2685.xml emd-2685.xml | 9.7 KB 9.7 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-2685-GluK2-SYM.jpg EMD-2685-GluK2-SYM.jpg | 48.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2685 http://ftp.pdbj.org/pub/emdb/structures/EMD-2685 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2685 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2685 | HTTPS FTP |
-Related structure data
| Related structure data |  4uqqMC  2680C  2684C  2686C  2687C  2688C  2689C  4uq6C  4uqjC  4uqkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2685.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2685.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of GluK2 desensitized state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.406 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : GluAK2 with 2S,4R-4-methylglutamate
| Entire | Name: GluAK2 with 2S,4R-4-methylglutamate |
|---|---|
| Components |
|
-Supramolecule #1000: GluAK2 with 2S,4R-4-methylglutamate
| Supramolecule | Name: GluAK2 with 2S,4R-4-methylglutamate / type: sample / ID: 1000 / Number unique components: 2 |
|---|---|
| Molecular weight | Experimental: 400 KDa / Theoretical: 400 KDa |
-Macromolecule #1: GluK2
| Macromolecule | Name: GluK2 / type: protein_or_peptide / ID: 1 / Oligomeric state: Tetramer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) / synonym: Rat Rattus norvegicus (Norway rat) / synonym: Rat |
| Molecular weight | Experimental: 400 KDa / Theoretical: 400 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) / Recombinant cell: Sf9 / Recombinant plasmid: pFastBac1 Spodoptera frugiperda (fall armyworm) / Recombinant cell: Sf9 / Recombinant plasmid: pFastBac1 |
| Sequence | UniProtKB: Glutamate receptor ionotropic, kainate 2 |
-Macromolecule #2: 2S,4R-4-methylglutamate
| Macromolecule | Name: 2S,4R-4-methylglutamate / type: ligand / ID: 2 / Recombinant expression: No |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Chemical component information | 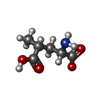 ChemComp-SYM: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.8 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 150 mM NaCl, 20 mM Tris pH 8.0, 0.75 mM DDM, 1 mM 2S,4R-4-methylglutamate |
| Grid | Details: Vitrified specimens were prepared by adding 2.5 uL of liganded protein at 1.8 mg/ml to R2/2 holey carbon grids (Quantifoil, Jena, Germany) rendered hydrophilic by chemical treatment to ...Details: Vitrified specimens were prepared by adding 2.5 uL of liganded protein at 1.8 mg/ml to R2/2 holey carbon grids (Quantifoil, Jena, Germany) rendered hydrophilic by chemical treatment to enable particle distribution into the holes (Meyerson JR, Rao P, Kumar K, Chittori S, Banerjee S, Pierson J, Mayer ML, and Subramaniam S, manuscript in preparation). |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 120 K / Instrument: FEI VITROBOT MARK IV / Method: Blot for 2 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 47000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.5 µm / Nominal defocus min: 2.0 µm / Nominal magnification: 47000 Bright-field microscopy / Nominal defocus max: 3.5 µm / Nominal defocus min: 2.0 µm / Nominal magnification: 47000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Date | Aug 1, 2013 |
| Image recording | Category: CCD / Film or detector model: FEI FALCON II (4k x 4k) / Number real images: 4837 / Average electron dose: 25 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: Each particle |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 7.6 Å / Resolution method: OTHER / Software - Name: Relion / Number images used: 21360 ) / Resolution.type: BY AUTHOR / Resolution: 7.6 Å / Resolution method: OTHER / Software - Name: Relion / Number images used: 21360 |
| Details | Particles were selected interactively at the computer terminal |
 Movie
Movie Controller
Controller













