[English] 日本語
 Yorodumi
Yorodumi- EMDB-8090: Cryo-EM structure of GluA2/3 AMPA receptor heterotetramer (model I) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8090 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
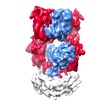
| Title | Cryo-EM structure of GluA2/3 AMPA receptor heterotetramer (model I) | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationchemical synaptic transmission, postsynaptic / Trafficking of AMPA receptors / Synaptic adhesion-like molecules / parallel fiber to Purkinje cell synapse / spine synapse / dendritic spine neck / dendritic spine head / Activation of AMPA receptors / response to lithium ion / perisynaptic space ...chemical synaptic transmission, postsynaptic / Trafficking of AMPA receptors / Synaptic adhesion-like molecules / parallel fiber to Purkinje cell synapse / spine synapse / dendritic spine neck / dendritic spine head / Activation of AMPA receptors / response to lithium ion / perisynaptic space / cellular response to glycine / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors /  immunoglobulin binding / AMPA glutamate receptor complex / kainate selective glutamate receptor activity / immunoglobulin binding / AMPA glutamate receptor complex / kainate selective glutamate receptor activity /  ionotropic glutamate receptor complex / extracellularly glutamate-gated ion channel activity / asymmetric synapse / regulation of receptor recycling / Unblocking of NMDA receptors, glutamate binding and activation / ionotropic glutamate receptor complex / extracellularly glutamate-gated ion channel activity / asymmetric synapse / regulation of receptor recycling / Unblocking of NMDA receptors, glutamate binding and activation /  glutamate receptor binding / regulation of postsynaptic membrane potential / glutamate receptor binding / regulation of postsynaptic membrane potential /  synaptic cleft / positive regulation of synaptic transmission / glutamate-gated receptor activity / presynaptic active zone membrane / response to fungicide / synaptic cleft / positive regulation of synaptic transmission / glutamate-gated receptor activity / presynaptic active zone membrane / response to fungicide /  regulation of synaptic transmission, glutamatergic / cellular response to brain-derived neurotrophic factor stimulus / somatodendritic compartment / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / regulation of synaptic transmission, glutamatergic / cellular response to brain-derived neurotrophic factor stimulus / somatodendritic compartment / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential /  ionotropic glutamate receptor binding / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / ionotropic glutamate receptor binding / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm /  cytoskeletal protein binding / monoatomic ion transmembrane transport / cytoskeletal protein binding / monoatomic ion transmembrane transport /  SNARE binding / dendritic shaft / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / SNARE binding / dendritic shaft / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential /  synaptic membrane / synaptic membrane /  synaptic transmission, glutamatergic / synaptic transmission, glutamatergic /  PDZ domain binding / postsynaptic density membrane / protein tetramerization / modulation of chemical synaptic transmission / Schaffer collateral - CA1 synapse / establishment of protein localization / PDZ domain binding / postsynaptic density membrane / protein tetramerization / modulation of chemical synaptic transmission / Schaffer collateral - CA1 synapse / establishment of protein localization /  terminal bouton / terminal bouton /  receptor internalization / synaptic vesicle membrane / cerebral cortex development / receptor internalization / synaptic vesicle membrane / cerebral cortex development /  synaptic vesicle / presynapse / synaptic vesicle / presynapse /  signaling receptor activity / signaling receptor activity /  presynaptic membrane / presynaptic membrane /  amyloid-beta binding / amyloid-beta binding /  growth cone / growth cone /  perikaryon / chemical synaptic transmission / perikaryon / chemical synaptic transmission /  postsynaptic membrane / postsynaptic membrane /  scaffold protein binding / scaffold protein binding /  dendritic spine / dendritic spine /  postsynaptic density / neuron projection / postsynaptic density / neuron projection /  axon / axon /  dendrite / neuronal cell body / glutamatergic synapse / dendrite / neuronal cell body / glutamatergic synapse /  synapse / protein-containing complex binding / endoplasmic reticulum membrane / synapse / protein-containing complex binding / endoplasmic reticulum membrane /  protein kinase binding / protein kinase binding /  cell surface / cell surface /  endoplasmic reticulum / protein-containing complex / endoplasmic reticulum / protein-containing complex /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | |||||||||
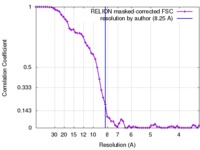
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 8.25 Å cryo EM / Resolution: 8.25 Å | |||||||||
 Authors Authors | Herguedas B / Garcia-Nafria J / Fernandez-Leiro R / Greger IH | |||||||||
 Citation Citation |  Journal: Science / Year: 2016 Journal: Science / Year: 2016Title: Structure and organization of heteromeric AMPA-type glutamate receptors. Authors: Beatriz Herguedas / Javier García-Nafría / Ondrej Cais / Rafael Fernández-Leiro / James Krieger / Hinze Ho / Ingo H Greger /  Abstract: AMPA-type glutamate receptors (AMPARs), which are central mediators of rapid neurotransmission and synaptic plasticity, predominantly exist as heteromers of the subunits GluA1 to GluA4. Here we ...AMPA-type glutamate receptors (AMPARs), which are central mediators of rapid neurotransmission and synaptic plasticity, predominantly exist as heteromers of the subunits GluA1 to GluA4. Here we report the first AMPAR heteromer structures, which deviate substantially from existing GluA2 homomer structures. Crystal structures of the GluA2/3 and GluA2/4 N-terminal domains reveal a novel compact conformation with an alternating arrangement of the four subunits around a central axis. This organization is confirmed by cysteine cross-linking in full-length receptors, and it permitted us to determine the structure of an intact GluA2/3 receptor by cryogenic electron microscopy. Two models in the ligand-free state, at resolutions of 8.25 and 10.3 angstroms, exhibit substantial vertical compression and close associations between domain layers, reminiscent of N-methyl-D-aspartate receptors. Model 1 resembles a resting state and model 2 a desensitized state, thus providing snapshots of gating transitions in the nominal absence of ligand. Our data reveal organizational features of heteromeric AMPARs and provide a framework to decipher AMPAR architecture and signaling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8090.map.gz emd_8090.map.gz | 2.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8090-v30.xml emd-8090-v30.xml emd-8090.xml emd-8090.xml | 22.5 KB 22.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8090_fsc.xml emd_8090_fsc.xml | 5.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_8090.png emd_8090.png | 122.2 KB | ||
| Masks |  emd_8090_msk_1.map emd_8090_msk_1.map emd_8090_msk_2.map emd_8090_msk_2.map | 16.8 MB 16.8 MB |  Mask map Mask map | |
| Others |  emd_8090_half_map_1.map.gz emd_8090_half_map_1.map.gz emd_8090_half_map_2.map.gz emd_8090_half_map_2.map.gz | 12.8 MB 12.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8090 http://ftp.pdbj.org/pub/emdb/structures/EMD-8090 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8090 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8090 | HTTPS FTP |
-Related structure data
| Related structure data |  5ideMC  8091C  5fwxC  5fwyC  5idfC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8090.map.gz / Format: CCP4 / Size: 16.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8090.map.gz / Format: CCP4 / Size: 16.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.76 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_8090_msk_1.map emd_8090_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
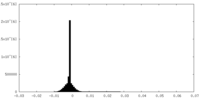
| Projections & Slices |
| ||||||||||||
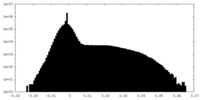
| Density Histograms |
-Mask #2
| File |  emd_8090_msk_2.map emd_8090_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: None
| File | emd_8090_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: None
| File | emd_8090_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : AMPA GluA2/3 heterotetramer
| Entire | Name: AMPA GluA2/3 heterotetramer |
|---|---|
| Components |
|
-Supramolecule #1: AMPA GluA2/3 heterotetramer
| Supramolecule | Name: AMPA GluA2/3 heterotetramer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Full-length GluA2/3 heterotetramer containing the A2_N292C and A3_265C mutations |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293 GntI- / Recombinant plasmid: prk5 and pIRES2-EGFP Homo sapiens (human) / Recombinant cell: HEK293 GntI- / Recombinant plasmid: prk5 and pIRES2-EGFP |
| Molecular weight | Theoretical: 400 KDa |
-Macromolecule #1: Glutamate receptor 2
| Macromolecule | Name: Glutamate receptor 2 / type: protein_or_peptide / ID: 1 Details: The sequence corresponds to mature rat GluA2 (residues 22-883, isoform Flip, edited at R/G and Q/R sites) with a Myc tag after the first residue and the N292C mutation Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Molecular weight | Theoretical: 97.663188 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: VEQKLISEED LSSNSIQIGG LFPRGADQEY SAFRVGMVQF STSEFRLTPH IDNLEVANSF AVTNAFCSQF SRGVYAIFGF YDKKSVNTI TSFCGTLHVS FITPSFPTDG THPFVIQMRP DLKGALLSLI EYYQWDKFAY LYDSDRGLST LQAVLDSAAE K KWQVTAIN ...String: VEQKLISEED LSSNSIQIGG LFPRGADQEY SAFRVGMVQF STSEFRLTPH IDNLEVANSF AVTNAFCSQF SRGVYAIFGF YDKKSVNTI TSFCGTLHVS FITPSFPTDG THPFVIQMRP DLKGALLSLI EYYQWDKFAY LYDSDRGLST LQAVLDSAAE K KWQVTAIN VGNINNDKKD ETYRSLFQDL ELKKERRVIL DCERDKVNDI VDQVITIGKH VKGYHYIIAN LGFTDGDLLK IQ FGGANVS GFQIVDYDDS LVSKFIERWS TLEEKEYPGA HTATIKYTSA LTYDAVQVMT EAFRCLRKQR IEISRRGNAG DCL ANPAVP WGQGVEIERA LKQVQVEGLS GNIKFDQNGK RINYTINIME LKTNGPRKIG YWSEVDKMVV TLTELPSGND TSGL ENKTV VVTTILESPY VMMKKNHEML EGNERYEGYC VDLAAEIAKH CGFKYKLTIV GDGKYGARDA DTKIWNGMVG ELVYG KADI AIAPLTITLV REEVIDFSKP FMSLGISIMI KKPQKSKPGV FSFLDPLAYE IWMCIVFAYI GVSVVLFLVS RFSPYE WHT EEFEDGRETQ SSESTNEFGI FNSLWFSLGA FMQQGCDISP RSLSGRIVGG VWWFFTLIII SSYTANLAAF LTVERMV SP IESAEDLSKQ TEIAYGTLDS GSTKEFFRRS KIAVFDKMWT YMRSAEPSVF VRTTAEGVAR VRKSKGKYAY LLESTMNE Y IEQRKPCDTM KVGGNLDSKG YGIATPKGSS LGTPVNLAVL KLSEQGVLDK LKNKWWYDKG ECGAKDSGSK EKTSALSLS NVAGVFYILV GGLGLAMLVA LIEFCYKSRA EAKRMKVAKN PQNINPSSSQ NSQNFATYKE GYNVYGIESV KI |
-Macromolecule #2: Glutamate receptor 3
| Macromolecule | Name: Glutamate receptor 3 / type: protein_or_peptide / ID: 2 Details: The sequence corresponds to the mature rat GluA3 subunit (residues 23-888, Flip isoform) with a Flag tag after the first residue and mutated at R439G and R265C Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Molecular weight | Theoretical: 99.075664 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GDYKDDDDKF PNTISIGGLF MRNTVQEHSA FRFAVQLYNT NQNTTEKPFH LNYHVDHLDS SNSFSVTNAF CSQFSRGVYA IFGFYDQMS MNTLTSFCGA LHTSFVTPSF PTDADVQFVI QMRPALKGAI LSLLSYYKWE KFVYLYDTER GFSVLQAIME A AVQNNWQV ...String: GDYKDDDDKF PNTISIGGLF MRNTVQEHSA FRFAVQLYNT NQNTTEKPFH LNYHVDHLDS SNSFSVTNAF CSQFSRGVYA IFGFYDQMS MNTLTSFCGA LHTSFVTPSF PTDADVQFVI QMRPALKGAI LSLLSYYKWE KFVYLYDTER GFSVLQAIME A AVQNNWQV TARSVGNIKD VQEFRRIIEE MDRRQEKRYL IDCEVERINT ILEQVVILGK HSRGYHYMLA NLGFTDILLE RV MHGGANI TGFQIVNNEN PMVQQFIQRW VRLDECEFPE AKNAPLKYTS ALTHDAILVI AEAFRYLRRQ RVDVSRRGSA GDC LANPAV PWSQGIDIER ALKMVQVQGM TGNIQFDTYG RRTNYTIDVY EMKVSGSRKA GYWNEYERFV PFSDQQISND SSSS ENRTI VVTTILESPY VMYKKNHEQL EGNERYEGYC VDLAYEIAKH VGIKYKLSIV GDGKYGARDP ETKIWNGMVG ELVYG RADI AVAPLTITLV REEVIDFSKP FMSLGISIMI KKPQKSKPGV FSFLDPLAYE IWMCIVFAYI GVSVVLFLVS RFSPYE WHL EDNNEEPRDP QSPPDPPNEF GIFNSLWFSL GAFMQQGCDI SPRSLSGRIV GGVWWFFTLI IISSYTANLA AFLTVER MV SPIESAEDLA KQTEIAYGTL DSGSTKEFFR RSKIAVYEKM WSYMKSAEPS VFTKTTADGV ARVRKSKGKF AFLLESTM N EYIEQRKPCD TMKVGGNLDS KGYGVATPKG SALGTPVNLA VLKLSEQGIL DKLKNKWWYD KGECGAKDSG SKDKTSALS LSNVAGVFYI LVGGLGLAMM VALIEFCYKS RAESKRMKLT KNTQNFKPAP ATNTQNYATY REGYNVYGTE SVKI |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.03 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 25 mM Tris pH 7.4, 0.25 % DDM, 150 mM NaCl |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Incubated for 1 minute, blotted for 3 seconds. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 28409 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm |
| Specialist optics | Energy filter - Name: GIF Quantum / Energy filter - Lower energy threshold: 0 eV / Energy filter - Upper energy threshold: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-20 / Number grids imaged: 2 / Number real images: 980 / Average exposure time: 25.0 sec. / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Details | For GluA2 chains (A,C), 2 copies of GluA2NTD (3HSY) and two copies of GluA2 LBD (1FTO) were fitted. For GluA3 chains (B,D), 2 copies of GluA3NTD (3O21) and two copies of GluA2 LBD (3UA8) were fitted.For the TMD region, the 4 chains of 3KG2 TMD(residues 509-544 594-629 784-817) were fitted as a rigid body. After fitting the sequence was corrected including mutations and side chains were removed. | ||||||||||||
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||||||||
| Output model |  PDB-5ide: |
 Movie
Movie Controller
Controller














 Z
Z Y
Y X
X