+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2853 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
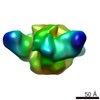
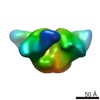
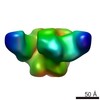
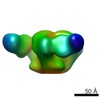
| Title | 3BNC117 Fab in complex with BG505 SOSIP.664 Trimer | |||||||||
 Map data Map data | Single particle reconstruction (20 Angstrom resolution based on FSC 0.5) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  HIV / HIV /  Fab / Env trimer Fab / Env trimer | |||||||||
| Biological species |    Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 20.0 Å negative staining / Resolution: 20.0 Å | |||||||||
 Authors Authors | Ozorowski G / Derking R / Sanders RW / Ward AB | |||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2015 Journal: PLoS Pathog / Year: 2015Title: Comprehensive antigenic map of a cleaved soluble HIV-1 envelope trimer. Authors: Ronald Derking / Gabriel Ozorowski / Kwinten Sliepen / Anila Yasmeen / Albert Cupo / Jonathan L Torres / Jean-Philippe Julien / Jeong Hyun Lee / Thijs van Montfort / Steven W de Taeye / Mark ...Authors: Ronald Derking / Gabriel Ozorowski / Kwinten Sliepen / Anila Yasmeen / Albert Cupo / Jonathan L Torres / Jean-Philippe Julien / Jeong Hyun Lee / Thijs van Montfort / Steven W de Taeye / Mark Connors / Dennis R Burton / Ian A Wilson / Per-Johan Klasse / Andrew B Ward / John P Moore / Rogier W Sanders /    Abstract: The trimeric envelope (Env) spike is the focus of vaccine design efforts aimed at generating broadly neutralizing antibodies (bNAbs) to protect against HIV-1 infection. Three recent developments have ...The trimeric envelope (Env) spike is the focus of vaccine design efforts aimed at generating broadly neutralizing antibodies (bNAbs) to protect against HIV-1 infection. Three recent developments have facilitated a thorough investigation of the antigenic structure of the Env trimer: 1) the isolation of many bNAbs against multiple different epitopes; 2) the generation of a soluble trimer mimic, BG505 SOSIP.664 gp140, that expresses most bNAb epitopes; 3) facile binding assays involving the oriented immobilization of tagged trimers. Using these tools, we generated an antigenic map of the trimer by antibody cross-competition. Our analysis delineates three well-defined epitope clusters (CD4 binding site, quaternary V1V2 and Asn332-centered oligomannose patch) and new epitopes at the gp120-gp41 interface. It also identifies the relationships among these clusters. In addition to epitope overlap, we defined three more ways in which antibodies can cross-compete: steric competition from binding to proximal but non-overlapping epitopes (e.g., PGT151 inhibition of 8ANC195 binding); allosteric inhibition (e.g., PGT145 inhibition of 1NC9, 8ANC195, PGT151 and CD4 binding); and competition by reorientation of glycans (e.g., PGT135 inhibition of CD4bs bNAbs, and CD4bs bNAb inhibition of 8ANC195). We further demonstrate that bNAb binding can be complex, often affecting several other areas of the trimer surface beyond the epitope. This extensive analysis of the antigenic structure and the epitope interrelationships of the Env trimer should aid in design of both bNAb-based therapies and vaccines intended to induce bNAbs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2853.map.gz emd_2853.map.gz | 14.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2853-v30.xml emd-2853-v30.xml emd-2853.xml emd-2853.xml | 11.1 KB 11.1 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-2853_3BNC117_BG505.png EMD-2853_3BNC117_BG505.png | 29.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2853 http://ftp.pdbj.org/pub/emdb/structures/EMD-2853 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2853 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2853 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2853.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2853.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Single particle reconstruction (20 Angstrom resolution based on FSC 0.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Fab of 3BNC117 monoclonal antibody bound to recombinant HIV-1 Env...
| Entire | Name: Fab of 3BNC117 monoclonal antibody bound to recombinant HIV-1 Env gp140 BG505 SOSIP.664 trimer |
|---|---|
| Components |
|
-Supramolecule #1000: Fab of 3BNC117 monoclonal antibody bound to recombinant HIV-1 Env...
| Supramolecule | Name: Fab of 3BNC117 monoclonal antibody bound to recombinant HIV-1 Env gp140 BG505 SOSIP.664 trimer type: sample / ID: 1000 Details: Size-exclusion chromatography purified gp140 trimers were used Oligomeric state: One Fab molecule binds to one BG505 SOSIP.664 gp140 monomer Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 570 KDa Method: Theoretical MW of Fab based on primary sequence; MW of trimer estimated using published values |
-Macromolecule #1: Soluble HIV-1 Envelope glycoprotein
| Macromolecule | Name: Soluble HIV-1 Envelope glycoprotein / type: protein_or_peptide / ID: 1 / Name.synonym: SOSIP Details: gp140 trimers were expressed recombinantly and purified using 2G12-affinity and size-exclusion chromatography Number of copies: 1 / Oligomeric state: Trimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 / Strain: BG505 / synonym: HIV-1 Human immunodeficiency virus 1 / Strain: BG505 / synonym: HIV-1 |
| Molecular weight | Theoretical: 420 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293F / Recombinant plasmid: pPPI4 Homo sapiens (human) / Recombinant cell: HEK293F / Recombinant plasmid: pPPI4 |
-Macromolecule #2: Fab of 3BNC117 human monoclonal antibody
| Macromolecule | Name: Fab of 3BNC117 human monoclonal antibody / type: protein_or_peptide / ID: 2 Details: Expressed recombinantly as IgG and cleaved into Fab using papain Number of copies: 1 / Oligomeric state: Dimer composed of heavy and light chain / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
| Molecular weight | Theoretical: 50 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant cell: HEK293F Homo sapiens (human) / Recombinant cell: HEK293F |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.01 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 50 mM Tris-HCl pH 7.4, 150 mM NaCl |
| Staining | Type: NEGATIVE Details: 3 microliters of 2% w/v uranyl formate were added to grids adsorbed with protein for 45 seconds and blotted |
| Grid | Details: 400 Cu mesh grid with carbon support, plasma cleaned |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 12 |
|---|---|
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Calibrated magnification: 52000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 52000 Bright-field microscopy / Nominal defocus max: 1.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 52000 |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC / Tilt angle min: -50 |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism corrected at 52,000x mag |
| Date | Sep 29, 2014 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Number real images: 145 / Average electron dose: 25 e/Å2 |
| Tilt angle max | 0 |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C3 (3 fold cyclic ) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: OTHER / Software - Name: EMAN2, SPARX / Number images used: 14956 ) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: OTHER / Software - Name: EMAN2, SPARX / Number images used: 14956 |
|---|---|
| Details | Particles were selected using an automatic selection program. A substack was created containing particles with 3 Fab molecules per trimer. An initial common-lines model was created using class averages and further refined using projection matching and an unbinned stack of particles. |
 Movie
Movie Controller
Controller