+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2373 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ribosome-RelA complex | |||||||||
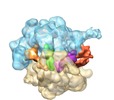
 Map data Map data | reconstruction of ribosome-RelA complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  ribosome / ribosome /  RelA / RelA /  stringent response stringent response | |||||||||
| Biological species |    Thermus thermophilus (bacteria) / Thermus thermophilus (bacteria) /   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / cryo EM /  negative staining / Resolution: 14.5 Å negative staining / Resolution: 14.5 Å | |||||||||
 Authors Authors | Agirrezabala X / Fernandez I / Kelley A / Gil-Carton D / Ramakrishnan V / Valle M | |||||||||
 Citation Citation |  Journal: EMBO Rep / Year: 2013 Journal: EMBO Rep / Year: 2013Title: The ribosome triggers the stringent response by RelA via a highly distorted tRNA. Authors: Xabier Agirrezabala / Israel S Fernández / Ann C Kelley / David Gil Cartón / Venki Ramakrishnan / Mikel Valle /  Abstract: The bacterial stringent response links nutrient starvation with the transcriptional control of genes. This process is initiated by the stringent factor RelA, which senses the presence of deacylated ...The bacterial stringent response links nutrient starvation with the transcriptional control of genes. This process is initiated by the stringent factor RelA, which senses the presence of deacylated tRNA in the ribosome as a symptom of amino-acid starvation to synthesize the alarmone (p)ppGpp. Here we report a cryo-EM study of RelA bound to ribosomes bearing cognate, deacylated tRNA in the A-site. The data show that RelA on the ribosome stabilizes an unusual distorted form of the tRNA, with the acceptor arm making contact with RelA and far from its normal location in the peptidyl transferase centre. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2373.map.gz emd_2373.map.gz | 33 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2373-v30.xml emd-2373-v30.xml emd-2373.xml emd-2373.xml | 11.6 KB 11.6 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-2373-4.tif EMD-2373-4.tif | 455.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2373 http://ftp.pdbj.org/pub/emdb/structures/EMD-2373 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2373 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2373 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2373.map.gz / Format: CCP4 / Size: 34.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2373.map.gz / Format: CCP4 / Size: 34.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | reconstruction of ribosome-RelA complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.75 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : 70S-RelA complex
| Entire | Name: 70S-RelA complex |
|---|---|
| Components |
|
-Supramolecule #1000: 70S-RelA complex
| Supramolecule | Name: 70S-RelA complex / type: sample / ID: 1000 / Number unique components: 5 |
|---|---|
| Molecular weight | Theoretical: 2.5 MDa |
-Supramolecule #1: 70S ribosome
| Supramolecule | Name: 70S ribosome / type: complex / ID: 1 / Recombinant expression: No / Ribosome-details: ribosome-prokaryote: ALL |
|---|---|
| Source (natural) | Organism:    Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
| Molecular weight | Theoretical: 2.5 MDa |
-Macromolecule #1: deacylated tRNA
| Macromolecule | Name: deacylated tRNA / type: rna / ID: 1 / Classification: OTHER / Structure: SINGLE STRANDED / Synthetic?: No |
|---|---|
| Source (natural) | Organism:    Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
-Macromolecule #2: tRNA
| Macromolecule | Name: tRNA / type: rna / ID: 2 / Classification: OTHER / Structure: SINGLE STRANDED / Synthetic?: No |
|---|---|
| Source (natural) | Organism:    Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
-Macromolecule #3: tRNA
| Macromolecule | Name: tRNA / type: rna / ID: 3 / Classification: OTHER / Structure: SINGLE STRANDED / Synthetic?: No |
|---|---|
| Source (natural) | Organism:    Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
-Macromolecule #4: RelA
| Macromolecule | Name: RelA / type: protein_or_peptide / ID: 4 / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Experimental details
-Structure determination
| Method |  negative staining, negative staining,  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.25 mg/mL |
|---|---|
| Buffer | pH: 7.45 Details: 5mM Hepes-KOH pH 7.45, 50mM KCl, 10mM NH4Cl, 10mM Mg-acetate, 5mM beta-mercapto-ethanol |
| Staining | Type: NEGATIVE / Details: cryo |
| Grid | Details: Quantifoil grids (2/4) with thin carbon on top |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 90 K / Instrument: FEI VITROBOT MARK I / Method: Blot for 3 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2200FS |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 40000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 40000 Bright-field microscopy / Cs: 2 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 40000 |
| Specialist optics | Energy filter - Name: in-column Omega filter / Energy filter - Lower energy threshold: 15.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Sample stage | Specimen holder model: GATAN LIQUID NITROGEN |
| Alignment procedure | Legacy - Astigmatism: corrected at 100k |
| Date | Jan 15, 2012 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 7 µm / Number real images: 425 / Average electron dose: 10 e/Å2 / Bits/pixel: 8 |
- Image processing
Image processing
| CTF correction | Details: defocus groups |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 14.5 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: XMIPP, SPIDER / Number images used: 28791 |
| Details | particles were selected by combining automated particle picking , multivariate data analysis and visual inspection |
-Atomic model buiding 1
| Initial model | PDB ID:  2wrn |
|---|---|
| Software | Name: MDFF |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
-Atomic model buiding 2
| Initial model | PDB ID:  2wro |
|---|---|
| Software | Name: MDFF |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
 Movie
Movie Controller
Controller