[English] 日本語
 Yorodumi
Yorodumi- EMDB-1268: Structure of bacteriophage SPP1 tail reveals trigger for DNA ejection. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1268 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of bacteriophage SPP1 tail reveals trigger for DNA ejection. | |||||||||
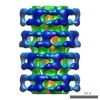
 Map data Map data | CCP4 map of the helical Tail of the bacteriophage SPP1 after DNA ejection | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Bacillus phage SPP1 (virus) Bacillus phage SPP1 (virus) | |||||||||
| Method | helical reconstruction /  negative staining / Resolution: 14.5 Å negative staining / Resolution: 14.5 Å | |||||||||
 Authors Authors | Plisson C / White HE / Auzat I / Sao-jose C / Lhuillier S / Tavares P / Orlova EV | |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2007 Journal: EMBO J / Year: 2007Title: Structure of bacteriophage SPP1 tail reveals trigger for DNA ejection. Authors: Celia Plisson / Helen E White / Isabelle Auzat / Amineh Zafarani / Carlos São-José / Sophie Lhuillier / Paulo Tavares / Elena V Orlova /  Abstract: The majority of known bacteriophages have long noncontractile tails (Siphoviridae) that serve as a pipeline for genome delivery into the host cytoplasm. The tail extremity distal from the phage head ...The majority of known bacteriophages have long noncontractile tails (Siphoviridae) that serve as a pipeline for genome delivery into the host cytoplasm. The tail extremity distal from the phage head is an adsorption device that recognises the bacterial receptor at the host cell surface. This interaction generates a signal transmitted to the head that leads to DNA release. We have determined structures of the bacteriophage SPP1 tail before and after DNA ejection. The results reveal extensive structural rearrangements in the internal wall of the tail tube. We propose that the adsorption device-receptor interaction triggers a conformational switch that is propagated as a domino-like cascade along the 1600 A-long helical tail structure to reach the head-to-tail connector. This leads to opening of the connector culminating in DNA exit from the head into the host cell through the tail tube. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1268.map.gz emd_1268.map.gz | 2.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1268-v30.xml emd-1268-v30.xml emd-1268.xml emd-1268.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| Images |  1268.gif 1268.gif | 31.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1268 http://ftp.pdbj.org/pub/emdb/structures/EMD-1268 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1268 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1268 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1268.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1268.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CCP4 map of the helical Tail of the bacteriophage SPP1 after DNA ejection | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.4 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Tail of the bacteriophage SPP1 after DNa ejection
| Entire | Name: Tail of the bacteriophage SPP1 after DNa ejection |
|---|---|
| Components |
|
-Supramolecule #1000: Tail of the bacteriophage SPP1 after DNa ejection
| Supramolecule | Name: Tail of the bacteriophage SPP1 after DNa ejection / type: sample / ID: 1000 / Oligomeric state: hexameric rings of gp17.1 and gp17.1star / Number unique components: 2 |
|---|
-Macromolecule #1: Major Tail Protein 1
| Macromolecule | Name: Major Tail Protein 1 / type: protein_or_peptide / ID: 1 / Name.synonym: gp17.1 Details: The 180 nm-long tail tube is composed of the two major tail proteins, gp17.1 and gp17.1star. They form a helical stack of 45 rings. Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Bacillus phage SPP1 (virus) / synonym: Bacteriophage SPP1 Bacillus phage SPP1 (virus) / synonym: Bacteriophage SPP1 |
| Molecular weight | Experimental: 19.1 KDa |
-Macromolecule #2: Major Tail Protein 2
| Macromolecule | Name: Major Tail Protein 2 / type: protein_or_peptide / ID: 2 / Name.synonym: gp17.1star / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Bacillus phage SPP1 (virus) / synonym: Bacteriophage SPP1 Bacillus phage SPP1 (virus) / synonym: Bacteriophage SPP1 |
| Molecular weight | Experimental: 28.2 KDa |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Details: 50 mM Tris-Cl, 300 mM NaCl, 5 mM MgCl2 |
|---|---|
| Staining | Type: NEGATIVE Details: 3.5 microliter of sample was applied onto carbon-coated copper grids, which were glow-discharged for 30 seconds. The sample was left for 1-2 minutes on the grids before blotting and staining ...Details: 3.5 microliter of sample was applied onto carbon-coated copper grids, which were glow-discharged for 30 seconds. The sample was left for 1-2 minutes on the grids before blotting and staining with a solution of 2% uranyl acetate. |
| Grid | Details: 300 mesh copper grid |
| Vitrification | Cryogen name: NONE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 50000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 50000 Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Gatan single tilt negative stain holder / Specimen holder model: OTHER |
| Temperature | Average: 298 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected at 100,000 times magnification |
| Details | Low Dose imaging |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 12 / Average electron dose: 10 e/Å2 / Od range: 2.5 / Bits/pixel: 8 |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: phase flipping |
|---|---|
| Final angle assignment | Details: IMAGIC beta range 65-115 degrees, gamma range 0-60 degrees |
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 40 Å Applied symmetry - Helical parameters - Δ&Phi: 21 ° Applied symmetry - Helical parameters - Axial symmetry: C6 (6 fold cyclic  ) )Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 14.5 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: IMAGIC, SPIDER / Details: exact filter back-projection |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















