[English] 日本語
 Yorodumi
Yorodumi- EMDB-8117: Structure of TRPV1 in complex with DkTx and RTX, determined in li... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8117 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of TRPV1 in complex with DkTx and RTX, determined in lipid nanodisc | |||||||||||||||
 Map data Map data | TRPV1 in complex with DkTx and RTX | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationtemperature-gated ion channel activity / response to capsazepine / excitatory extracellular ligand-gated monoatomic ion channel activity / negative regulation of establishment of blood-brain barrier / sensory perception of mechanical stimulus / peptide secretion / urinary bladder smooth muscle contraction / detection of chemical stimulus involved in sensory perception of pain / cellular response to temperature stimulus / smooth muscle contraction involved in micturition ...temperature-gated ion channel activity / response to capsazepine / excitatory extracellular ligand-gated monoatomic ion channel activity / negative regulation of establishment of blood-brain barrier / sensory perception of mechanical stimulus / peptide secretion / urinary bladder smooth muscle contraction / detection of chemical stimulus involved in sensory perception of pain / cellular response to temperature stimulus / smooth muscle contraction involved in micturition /  TRP channels / cellular response to acidic pH / TRP channels / cellular response to acidic pH /  thermoception / fever generation / detection of temperature stimulus involved in thermoception / glutamate secretion / dendritic spine membrane / negative regulation of systemic arterial blood pressure / chloride channel regulator activity / response to pH / ion channel regulator activity / monoatomic cation transmembrane transporter activity / cellular response to ATP / response to pain / thermoception / fever generation / detection of temperature stimulus involved in thermoception / glutamate secretion / dendritic spine membrane / negative regulation of systemic arterial blood pressure / chloride channel regulator activity / response to pH / ion channel regulator activity / monoatomic cation transmembrane transporter activity / cellular response to ATP / response to pain /  temperature homeostasis / negative regulation of heart rate / cellular response to alkaloid / diet induced thermogenesis / behavioral response to pain / extracellular ligand-gated monoatomic ion channel activity / intracellularly gated calcium channel activity / cellular response to cytokine stimulus / calcium ion import across plasma membrane / negative regulation of mitochondrial membrane potential / detection of temperature stimulus involved in sensory perception of pain / ligand-gated monoatomic ion channel activity / monoatomic cation channel activity / sensory perception of pain / response to organonitrogen compound / monoatomic ion transmembrane transport / temperature homeostasis / negative regulation of heart rate / cellular response to alkaloid / diet induced thermogenesis / behavioral response to pain / extracellular ligand-gated monoatomic ion channel activity / intracellularly gated calcium channel activity / cellular response to cytokine stimulus / calcium ion import across plasma membrane / negative regulation of mitochondrial membrane potential / detection of temperature stimulus involved in sensory perception of pain / ligand-gated monoatomic ion channel activity / monoatomic cation channel activity / sensory perception of pain / response to organonitrogen compound / monoatomic ion transmembrane transport /  phosphatidylinositol binding / cellular response to nerve growth factor stimulus / calcium ion transmembrane transport / phosphatidylinositol binding / cellular response to nerve growth factor stimulus / calcium ion transmembrane transport /  phosphoprotein binding / microglial cell activation / phosphoprotein binding / microglial cell activation /  calcium channel activity / lipid metabolic process / response to peptide hormone / cellular response to growth factor stimulus / calcium ion transport / positive regulation of nitric oxide biosynthetic process / transmembrane signaling receptor activity / cellular response to heat / cellular response to tumor necrosis factor / positive regulation of cytosolic calcium ion concentration / response to heat / calcium channel activity / lipid metabolic process / response to peptide hormone / cellular response to growth factor stimulus / calcium ion transport / positive regulation of nitric oxide biosynthetic process / transmembrane signaling receptor activity / cellular response to heat / cellular response to tumor necrosis factor / positive regulation of cytosolic calcium ion concentration / response to heat /  toxin activity / toxin activity /  postsynaptic membrane / protein homotetramerization / postsynaptic membrane / protein homotetramerization /  calmodulin binding / neuron projection / positive regulation of apoptotic process / external side of plasma membrane / calmodulin binding / neuron projection / positive regulation of apoptotic process / external side of plasma membrane /  dendrite / neuronal cell body / dendrite / neuronal cell body /  lipid binding / negative regulation of transcription by RNA polymerase II / extracellular region / lipid binding / negative regulation of transcription by RNA polymerase II / extracellular region /  ATP binding / ATP binding /  membrane / identical protein binding / membrane / identical protein binding /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||||||||
| Biological species |   Rattus norvegicus (Norway rat) / Rattus norvegicus (Norway rat) /   Haplopelma schmidti (Chinese earth tiger) Haplopelma schmidti (Chinese earth tiger) | |||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.95 Å cryo EM / Resolution: 2.95 Å | |||||||||||||||
 Authors Authors | Gao Y / Cao E / Julius D / Cheng Y | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2016 Journal: Nature / Year: 2016Title: TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Authors: Yuan Gao / Erhu Cao / David Julius / Yifan Cheng /  Abstract: When integral membrane proteins are visualized in detergents or other artificial systems, an important layer of information is lost regarding lipid interactions and their effects on protein structure. ...When integral membrane proteins are visualized in detergents or other artificial systems, an important layer of information is lost regarding lipid interactions and their effects on protein structure. This is especially relevant to proteins for which lipids have both structural and regulatory roles. Here we demonstrate the power of combining electron cryo-microscopy with lipid nanodisc technology to ascertain the structure of the rat TRPV1 ion channel in a native bilayer environment. Using this approach, we determined the locations of annular and regulatory lipids and showed that specific phospholipid interactions enhance binding of a spider toxin to TRPV1 through formation of a tripartite complex. Furthermore, phosphatidylinositol lipids occupy the binding site for capsaicin and other vanilloid ligands, suggesting a mechanism whereby chemical or thermal stimuli elicit channel activation by promoting the release of bioactive lipids from a critical allosteric regulatory site. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8117.map.gz emd_8117.map.gz | 24.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8117-v30.xml emd-8117-v30.xml emd-8117.xml emd-8117.xml | 26.6 KB 26.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8117_fsc.xml emd_8117_fsc.xml | 6.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_8117.png emd_8117.png | 209.8 KB | ||
| Others |  emd_8117_half_map_1.map.gz emd_8117_half_map_1.map.gz emd_8117_half_map_2.map.gz emd_8117_half_map_2.map.gz | 17.8 MB 17.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8117 http://ftp.pdbj.org/pub/emdb/structures/EMD-8117 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8117 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8117 | HTTPS FTP |
-Related structure data
| Related structure data |  5irxMC  8118C  8119C  8120C  5irzC  5is0C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10059 (Title: Structure of TRPV1 in complex with DkTx and RTX, determined in lipid nanodisc EMPIAR-10059 (Title: Structure of TRPV1 in complex with DkTx and RTX, determined in lipid nanodiscData size: 93.8 Data #1: Motion corrected, dose-weighted sum of micrographs of TRPV1-DkTx/RTX embedded in lipid nanodisc [micrographs - single frame] Data #2: Raw particle image stack of TRPV1-DkTx/RTX embedded in lipid nanodisc [picked particles - multiframe - unprocessed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8117.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8117.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TRPV1 in complex with DkTx and RTX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.2156 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: TRPV1 in complex with DkTx and RTX, half map 1
| File | emd_8117_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TRPV1 in complex with DkTx and RTX, half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: TRPV1 in complex with DkTx and RTX, half map 2
| File | emd_8117_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TRPV1 in complex with DkTx and RTX, half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TRPV1 ion channel in complex with DkTx and RTX
| Entire | Name: TRPV1 ion channel in complex with DkTx and RTX |
|---|---|
| Components |
|
-Supramolecule #1: TRPV1 ion channel in complex with DkTx and RTX
| Supramolecule | Name: TRPV1 ion channel in complex with DkTx and RTX / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: Two molecules of DkTx and 4 molecules of RTX bound to one TRPV1 homo-tetramer |
|---|
-Supramolecule #2: Transient receptor potential cation channel subfamily V member 1
| Supramolecule | Name: Transient receptor potential cation channel subfamily V member 1 type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Recombinant expression | Organism:   Homo sapiens (human) / Recombinant plasmid: unknown Homo sapiens (human) / Recombinant plasmid: unknown |
-Supramolecule #3: Tau-theraphotoxin-Hs1a
| Supramolecule | Name: Tau-theraphotoxin-Hs1a / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:   Haplopelma schmidti (Chinese earth tiger) Haplopelma schmidti (Chinese earth tiger) |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) / Recombinant plasmid: unknown Escherichia coli BL21(DE3) (bacteria) / Recombinant plasmid: unknown |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 1
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 1 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Molecular weight | Theoretical: 72.959297 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AMGSRLYDRR SIFDAVAQSN CQELESLLPF LQRSKKRLTD SEFKDPETGK TCLLKAMLNL HNGQNDTIAL LLDVARKTDS LKQFVNASY TDSYYKGQTA LHIAIERRNM TLVTLLVENG ADVQAAANGD FFKKTKGRPG FYFGELPLSL AACTNQLAIV K FLLQNSWQ ...String: AMGSRLYDRR SIFDAVAQSN CQELESLLPF LQRSKKRLTD SEFKDPETGK TCLLKAMLNL HNGQNDTIAL LLDVARKTDS LKQFVNASY TDSYYKGQTA LHIAIERRNM TLVTLLVENG ADVQAAANGD FFKKTKGRPG FYFGELPLSL AACTNQLAIV K FLLQNSWQ PADISARDSV GNTVLHALVE VADNTVDNTK FVTSMYNEIL ILGAKLHPTL KLEEITNRKG LTPLALAASS GK IGVLAYI LQREIHEPEC RHLSRKFTEW AYGPVHSSLY DLSCIDTCEK NSVLEVIAYS SSETPNRHDM LLVEPLNRLL QDK WDRFVK RIFYFNFFVY CLYMIIFTAA AYYRPVEGLP PYKLKNTVGD YFRVTGEILS VSGGVYFFFR GIQYFLQRRP SLKS LFVDS YSEILFFVQS LFMLVSVVLY FSQRKEYVAS MVFSLAMGWT NMLYYTRGFQ QMGIYAVMIE KMILRDLCRF MFVYL VFLF GFSTAVVTLI EDGKYNSLYS TCLELFKFTI GMGDLEFTEN YDFKAVFIIL LLAYVILTYI LLLNMLIALM GETVNK IAQ ESKNIWKLQR AITILDTEKS FLKCMRKAFR SGKLLQVGFT PDGKDDYRWC FRVDEVNWTT WNTNVGIINE DPG |
-Macromolecule #2: Tau-theraphotoxin-Hs1a
| Macromolecule | Name: Tau-theraphotoxin-Hs1a / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Haplopelma schmidti (Chinese earth tiger) Haplopelma schmidti (Chinese earth tiger) |
| Molecular weight | Theoretical: 8.552988 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: DCAKEGEVCS WGKKCCDLDN FYCPMEFIPH CKKYKPYVPV TTNCAKEGEV CGWGSKCCHG LDCPLAFIPY CEKYR |
-Macromolecule #3: (4R,7S)-4-hydroxy-N,N,N-trimethyl-4,9-dioxo-7-[(pentanoyloxy)meth...
| Macromolecule | Name: (4R,7S)-4-hydroxy-N,N,N-trimethyl-4,9-dioxo-7-[(pentanoyloxy)methyl]-3,5,8-trioxa-4lambda~5~-phosphatetradecan-1-aminium type: ligand / ID: 3 / Number of copies: 4 / Formula: 6O8 |
|---|---|
| Molecular weight | Theoretical: 440.489 Da |
| Chemical component information | 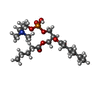 ChemComp-6O8: |
-Macromolecule #4: (2S)-3-{[(S)-(2-aminoethoxy)(hydroxy)phosphoryl]oxy}-2-(hexanoylo...
| Macromolecule | Name: (2S)-3-{[(S)-(2-aminoethoxy)(hydroxy)phosphoryl]oxy}-2-(hexanoyloxy)propyl hexanoate type: ligand / ID: 4 / Number of copies: 8 / Formula: 6OE |
|---|---|
| Molecular weight | Theoretical: 411.428 Da |
| Chemical component information | 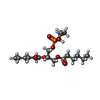 ChemComp-6OE: |
-Macromolecule #5: resiniferatoxin
| Macromolecule | Name: resiniferatoxin / type: ligand / ID: 5 / Number of copies: 4 / Formula: 6EU |
|---|---|
| Molecular weight | Theoretical: 628.708 Da |
| Chemical component information | 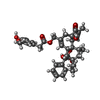 ChemComp-6EU: |
-Macromolecule #6: (2S)-2-(acetyloxy)-3-{[(R)-(2-aminoethoxy)(hydroxy)phosphoryl]oxy...
| Macromolecule | Name: (2S)-2-(acetyloxy)-3-{[(R)-(2-aminoethoxy)(hydroxy)phosphoryl]oxy}propyl pentanoate type: ligand / ID: 6 / Number of copies: 4 / Formula: 6O9 |
|---|---|
| Molecular weight | Theoretical: 341.295 Da |
| Chemical component information |  ChemComp-6O9: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 12.0 nm / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 290.0 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK III Details: Blot for 6 seconds before plunging into liquid ethane (FEI VITROBOT MARK III).. | ||||||||||||
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 30.0 µm / Calibrated defocus max: 2.2 µm / Calibrated defocus min: 0.7 µm / Calibrated magnification: 41132 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.7 µm / Nominal magnification: 31000 Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.7 µm / Nominal magnification: 31000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
| Temperature | Min: 70.0 K / Max: 70.0 K |
| Details | Grid screening was performed manually. |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-30 / Number grids imaged: 1 / Number real images: 1200 / Average exposure time: 6.0 sec. / Average electron dose: 41.0 e/Å2 Details: Images were collected in movie mode at 5 frames per second for 6 seconds. |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)







































