+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6183 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
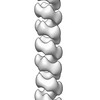
| Title | 3D structure of RepA-WH1 single filaments | |||||||||
 Map data Map data | Negative-staining EM reconstruction of single repA-WH1 filament | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RepA-WH1 prionoid / amyloid protofilaments /  amyloid assembly amyloid assembly | |||||||||
| Biological species |   Pseudomonas aeruginosa (bacteria) Pseudomonas aeruginosa (bacteria) | |||||||||
| Method | helical reconstruction /  negative staining / Resolution: 29.0 Å negative staining / Resolution: 29.0 Å | |||||||||
 Authors Authors | Torreira E / Moreno M / Fuentes-Perez ME / Fernandez C / Martin-Benito J / Moreno-Herrero F / Giraldo R / Llorca O | |||||||||
 Citation Citation |  Journal: Structure / Year: 2015 Journal: Structure / Year: 2015Title: Amyloidogenesis of bacterial prionoid RepA-WH1 recapitulates dimer to monomer transitions of RepA in DNA replication initiation. Authors: Eva Torreira / María Moreno-Del Álamo / Maria Eugenia Fuentes-Perez / Cristina Fernández / Jaime Martín-Benito / Fernando Moreno-Herrero / Rafael Giraldo / Oscar Llorca /  Abstract: Most available structures of amyloids correspond to peptide fragments that self-assemble in extended cross β sheets. However, structures in which a whole protein domain acts as building block of an ...Most available structures of amyloids correspond to peptide fragments that self-assemble in extended cross β sheets. However, structures in which a whole protein domain acts as building block of an amyloid fiber are scarce, in spite of their relevance to understand amyloidogenesis. Here, we use electron microscopy (EM) and atomic force microscopy (AFM) to analyze the structure of amyloid filaments assembled by RepA-WH1, a winged-helix domain from a DNA replication initiator in bacterial plasmids. RepA-WH1 functions as a cytotoxic bacterial prionoid that recapitulates features of mammalian amyloid proteinopathies. RepA are dimers that monomerize at the origin to initiate replication, and we find that RepA-WH1 reproduces this transition to form amyloids. RepA-WH1 assembles double helical filaments by lateral association of a single-stranded precursor built by monomers. Double filaments then associate in mature fibers. The intracellular and cytotoxic RepA-WH1 aggregates might reproduce the hierarchical assembly of human amyloidogenic proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6183.map.gz emd_6183.map.gz | 3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6183-v30.xml emd-6183-v30.xml emd-6183.xml emd-6183.xml | 10.3 KB 10.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6183.jpg emd_6183.jpg | 456.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6183 http://ftp.pdbj.org/pub/emdb/structures/EMD-6183 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6183 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6183 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6183.map.gz / Format: CCP4 / Size: 3.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6183.map.gz / Format: CCP4 / Size: 3.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative-staining EM reconstruction of single repA-WH1 filament | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.75 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Single RepA-WH1 filament
| Entire | Name: Single RepA-WH1 filament |
|---|---|
| Components |
|
-Supramolecule #1000: Single RepA-WH1 filament
| Supramolecule | Name: Single RepA-WH1 filament / type: sample / ID: 1000 / Oligomeric state: single-chain helical filament / Number unique components: 1 |
|---|
-Macromolecule #1: RepA
| Macromolecule | Name: RepA / type: protein_or_peptide / ID: 1 / Oligomeric state: single-chain helical filament / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Pseudomonas aeruginosa (bacteria) Pseudomonas aeruginosa (bacteria) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Details: 0.1 M Na2SO4, 40 mM HEPES, 5 mM MgSO4, 7% PEG4000, 3% MPD |
|---|---|
| Staining | Type: NEGATIVE Details: Specimens were adsorbed on glow-discharged copper grids and stained using 2% uranyl acetate for 2 minutes. |
| Grid | Details: 400-mesh copper grids |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 1230 |
|---|---|
| Electron beam | Acceleration voltage: 100 kV / Electron source: TUNGSTEN HAIRPIN |
| Electron optics | Calibrated magnification: 41586 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 41586 Bright-field microscopy / Nominal magnification: 41586 |
| Sample stage | Specimen holder model: JEOL |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected using a TVIPS F416 CMOS and the EM-MENU software (TVIPS). |
| Date | Sep 1, 2012 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Digitization - Sampling interval: 15.6 µm / Average electron dose: 18 e/Å2 / Bits/pixel: 16 |
- Image processing
Image processing
| CTF correction | Details: Each micrograph using BSOFT |
|---|---|
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 12.5 Å Applied symmetry - Helical parameters - Δ&Phi: 81 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 29.0 Å / Resolution method: OTHER |
| Details | The particles were 2D-classified using CL2D implemented in Xmipp, and further processed using IHRSR. Note: Due to map resolution, the hand is arbitrary. |
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Fitting was carried out both in individual segments and in the whole filament. In this case, helical symmetry was further applied to fill the EM map. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller