+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5844 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
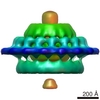
| Title | Subtomogram average of a virus-associated pyramid | |||||||||
 Map data Map data | Reconstruction of a virus associated pyramid | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | VAP / archaeovirus /  archaea / SIRV2 / Sulfolobus islandicus / viral egress archaea / SIRV2 / Sulfolobus islandicus / viral egress | |||||||||
| Function / homology | : / membrane => GO:0016020 / Uncharacterized protein Function and homology information Function and homology information | |||||||||
| Biological species |    Sulfolobus islandicus rod-shaped virus 2 Sulfolobus islandicus rod-shaped virus 2 | |||||||||
| Method | subtomogram averaging /  cryo EM cryo EM | |||||||||
 Authors Authors | Daum B / Kuehlbrandt W | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2014 Journal: Proc Natl Acad Sci U S A / Year: 2014Title: Self-assembly of the general membrane-remodeling protein PVAP into sevenfold virus-associated pyramids. Authors: Bertram Daum / Tessa E F Quax / Martin Sachse / Deryck J Mills / Julia Reimann / Özkan Yildiz / Sabine Häder / Cosmin Saveanu / Patrick Forterre / Sonja-Verena Albers / Werner Kühlbrandt ...Authors: Bertram Daum / Tessa E F Quax / Martin Sachse / Deryck J Mills / Julia Reimann / Özkan Yildiz / Sabine Häder / Cosmin Saveanu / Patrick Forterre / Sonja-Verena Albers / Werner Kühlbrandt / David Prangishvili /  Abstract: Viruses have developed a wide range of strategies to escape from the host cells in which they replicate. For egress some archaeal viruses use a pyramidal structure with sevenfold rotational symmetry. ...Viruses have developed a wide range of strategies to escape from the host cells in which they replicate. For egress some archaeal viruses use a pyramidal structure with sevenfold rotational symmetry. Virus-associated pyramids (VAPs) assemble in the host cell membrane from the virus-encoded protein PVAP and open at the end of the infection cycle. We characterize this unusual supramolecular assembly using a combination of genetic, biochemical, and electron microscopic techniques. By whole-cell electron cryotomography, we monitored morphological changes in virus-infected host cells. Subtomogram averaging reveals the VAP structure. By heterologous expression of PVAP in cells from all three domains of life, we demonstrate that the protein integrates indiscriminately into virtually any biological membrane, where it forms sevenfold pyramids. We identify the protein domains essential for VAP formation in PVAP truncation mutants by their ability to remodel the cell membrane. Self-assembly of PVAP into pyramids requires at least two different, in-plane and out-of-plane, protein interactions. Our findings allow us to propose a model describing how PVAP arranges to form sevenfold pyramids and suggest how this small, robust protein may be used as a general membrane-remodeling system. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5844.map.gz emd_5844.map.gz | 2.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5844-v30.xml emd-5844-v30.xml emd-5844.xml emd-5844.xml | 10.3 KB 10.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5844_1.jpg emd_5844_1.jpg emd_5844_2.tif emd_5844_2.tif | 124.9 KB 2.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5844 http://ftp.pdbj.org/pub/emdb/structures/EMD-5844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5844 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5844.map.gz / Format: CCP4 / Size: 3.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5844.map.gz / Format: CCP4 / Size: 3.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of a virus associated pyramid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 8.663 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Reconstruction of a virus-associated pyramid induced by overexpre...
| Entire | Name: Reconstruction of a virus-associated pyramid induced by overexpressing the viral protein PVAP in E. coli |
|---|---|
| Components |
|
-Supramolecule #1000: Reconstruction of a virus-associated pyramid induced by overexpre...
| Supramolecule | Name: Reconstruction of a virus-associated pyramid induced by overexpressing the viral protein PVAP in E. coli type: sample / ID: 1000 / Number unique components: 1 |
|---|
-Macromolecule #1: protein forming virus-associated pyramids
| Macromolecule | Name: protein forming virus-associated pyramids / type: protein_or_peptide / ID: 1 / Name.synonym: P98, PVAP Details: The gene encoding PVAP was heterologously overexpressed in E. coli to incorporate pyramid-shaped multimers of PVAP into the plasma membrane. Oligomeric state: Multimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:    Sulfolobus islandicus rod-shaped virus 2 / Location in cell: Plasma membrane Sulfolobus islandicus rod-shaped virus 2 / Location in cell: Plasma membrane |
| Molecular weight | Experimental: 10 KDa / Theoretical: 10 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) / Recombinant strain: Rosetta(DE3)pLys / Recombinant plasmid: pSA4 Escherichia coli (E. coli) / Recombinant strain: Rosetta(DE3)pLys / Recombinant plasmid: pSA4 |
| Sequence | UniProtKB: Uncharacterized protein / GO: GO: 0951408 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 / Details: 50 mM Tris, 300 mM NaCl |
|---|---|
| Grid | Details: Quantifoil 300 mesh R2/2, glow-discharged |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 10 % / Instrument: HOMEMADE PLUNGER Method: Blot for 3-5 seconds from the side where the sample was applied. |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 16870 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.2 mm / Nominal defocus max: 10.0 µm / Nominal defocus min: 8.0 µm / Nominal magnification: 27500 Bright-field microscopy / Cs: 2.2 mm / Nominal defocus max: 10.0 µm / Nominal defocus min: 8.0 µm / Nominal magnification: 27500 |
| Specialist optics | Energy filter - Name: Gatan Tridium GIF |
| Sample stage | Specimen holder: liquid nitrogen cooled / Specimen holder model: OTHER / Tilt series - Axis1 - Min angle: -60.73 ° / Tilt series - Axis1 - Max angle: 59.20 ° |
| Temperature | Min: 89 K / Max: 96 K / Average: 90 K |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 40,000 times magnification |
| Date | Mar 12, 2012 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 1000 (2k x 2k) / Bits/pixel: 32 |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Software - Name: PEET, IMOD / Number subtomograms used: 57 |
|---|
 Movie
Movie Controller
Controller