+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5691 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
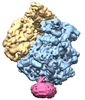
| Title | Structure of the SecY protein translocation channel in action | |||||||||
 Map data Map data | Reconstruction of archaeal 70S ribosome-SecYEbeta complex from Methanococcus jannaschii | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | archaeal 70S ribosome / SecYEbeta channel / co-translational translocation | |||||||||
| Function / homology |  Function and homology information Function and homology information intracellular protein transmembrane transport / SRP-dependent cotranslational protein targeting to membrane, translocation / intracellular protein transmembrane transport / SRP-dependent cotranslational protein targeting to membrane, translocation /  signal sequence binding / protein transmembrane transporter activity / signal sequence binding / protein transmembrane transporter activity /  protein secretion / protein secretion /  protein targeting / protein targeting /  protein transport / protein transport /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |    Methanocaldococcus jannaschii (archaea) Methanocaldococcus jannaschii (archaea) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 9.0 Å cryo EM / Resolution: 9.0 Å | |||||||||
 Authors Authors | Park P / Menetret JF / Gumbart JC / Ludtke SJ / Li W / Whynot A / Rapoport TA / Akey CW | |||||||||
 Citation Citation |  Journal: Nature / Year: 2014 Journal: Nature / Year: 2014Title: Structure of the SecY channel during initiation of protein translocation. Authors: Eunyong Park / Jean-François Ménétret / James C Gumbart / Steven J Ludtke / Weikai Li / Andrew Whynot / Tom A Rapoport / Christopher W Akey /  Abstract: Many secretory proteins are targeted by signal sequences to a protein-conducting channel, formed by prokaryotic SecY or eukaryotic Sec61 complexes, and are translocated across the membrane during ...Many secretory proteins are targeted by signal sequences to a protein-conducting channel, formed by prokaryotic SecY or eukaryotic Sec61 complexes, and are translocated across the membrane during their synthesis. Crystal structures of the inactive channel show that the SecY subunit of the heterotrimeric complex consists of two halves that form an hourglass-shaped pore with a constriction in the middle of the membrane and a lateral gate that faces the lipid phase. The closed channel has an empty cytoplasmic funnel and an extracellular funnel that is filled with a small helical domain, called the plug. During initiation of translocation, a ribosome-nascent chain complex binds to the SecY (or Sec61) complex, resulting in insertion of the nascent chain. However, the mechanism of channel opening during translocation is unclear. Here we have addressed this question by determining structures of inactive and active ribosome-channel complexes with cryo-electron microscopy. Non-translating ribosome-SecY channel complexes derived from Methanocaldococcus jannaschii or Escherichia coli show the channel in its closed state, and indicate that ribosome binding per se causes only minor changes. The structure of an active E. coli ribosome-channel complex demonstrates that the nascent chain opens the channel, causing mostly rigid body movements of the amino- and carboxy-terminal halves of SecY. In this early translocation intermediate, the polypeptide inserts as a loop into the SecY channel with the hydrophobic signal sequence intercalated into the open lateral gate. The nascent chain also forms a loop on the cytoplasmic surface of SecY rather than entering the channel directly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5691.map.gz emd_5691.map.gz | 1.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5691-v30.xml emd-5691-v30.xml emd-5691.xml emd-5691.xml | 26.2 KB 26.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5691.jpg emd_5691.jpg | 164.1 KB | ||
| Masks |  emd_5691_msk_1.map emd_5691_msk_1.map emd_5691_msk_2.map emd_5691_msk_2.map emd_5691_msk_3.map emd_5691_msk_3.map emd_5691_msk_4.map emd_5691_msk_4.map emd_5691_msk_5.map emd_5691_msk_5.map | 16.4 MB 16.4 MB 160.8 KB 16.4 MB 2.2 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5691 http://ftp.pdbj.org/pub/emdb/structures/EMD-5691 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5691 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5691 | HTTPS FTP |
-Related structure data
| Related structure data |  4v4nMC  5692C  5693C  3j45C  3j46C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_5691.map.gz / Format: CCP4 / Size: 16 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5691.map.gz / Format: CCP4 / Size: 16 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of archaeal 70S ribosome-SecYEbeta complex from Methanococcus jannaschii | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.73 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Segmentation: zone large ribosomal subunit
| Annotation | zone large ribosomal subunit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_5691_msk_1.map emd_5691_msk_1.map | ||||||||||||
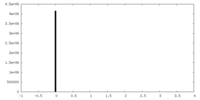
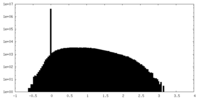
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Segmentation: zoned full channel desnity
| Annotation | zoned full channel desnity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_5691_msk_2.map emd_5691_msk_2.map | ||||||||||||
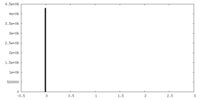
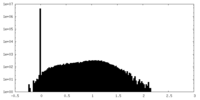
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Segmentation: segmented density with only SecYEbeta
| Annotation | segmented density with only SecYEbeta | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_5691_msk_3.map emd_5691_msk_3.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Segmentation: zoned micelle
| Annotation | zoned micelle | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_5691_msk_4.map emd_5691_msk_4.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Segmentation: zoned small ribosomal subunit
| Annotation | zoned small ribosomal subunit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_5691_msk_5.map emd_5691_msk_5.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Methanococcus jannaschii 70S ribosome-SecYEbeta complex
| Entire | Name: Methanococcus jannaschii 70S ribosome-SecYEbeta complex |
|---|---|
| Components |
|
-Supramolecule #1000: Methanococcus jannaschii 70S ribosome-SecYEbeta complex
| Supramolecule | Name: Methanococcus jannaschii 70S ribosome-SecYEbeta complex type: sample / ID: 1000 Details: The sample was reconstituted from ribosomal subunits purified from M. jannaschii and from recombinant SecYEbeta. Oligomeric state: one 70S, one E-site tRNA, one SecYEbeta channel Number unique components: 3 |
|---|---|
| Molecular weight | Theoretical: 2.6 MDa |
-Supramolecule #1: non-translating 70S ribosome
| Supramolecule | Name: non-translating 70S ribosome / type: complex / ID: 1 / Recombinant expression: No / Database: NCBI / Ribosome-details: ribosome-prokaryote: ALL |
|---|---|
| Source (natural) | Organism:    Methanocaldococcus jannaschii (archaea) / Location in cell: cytoplasm Methanocaldococcus jannaschii (archaea) / Location in cell: cytoplasm |
| Molecular weight | Theoretical: 2.5 MDa |
-Macromolecule #1: SecYEbeta
| Macromolecule | Name: SecYEbeta / type: protein_or_peptide / ID: 1 / Name.synonym: SecY channel / Details: SecY: Q60175.2, SecE: Q57817.1, Secbeta: P60460.1 / Number of copies: 1 / Oligomeric state: heterotrimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:    Methanocaldococcus jannaschii (archaea) / Location in cell: inner membrane Methanocaldococcus jannaschii (archaea) / Location in cell: inner membrane |
| Molecular weight | Theoretical: 61.8 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) / Recombinant strain: C43(DE3) / Recombinant plasmid: pBAD22 Escherichia coli (E. coli) / Recombinant strain: C43(DE3) / Recombinant plasmid: pBAD22 |
-Macromolecule #2: transfer RNA
| Macromolecule | Name: transfer RNA / type: rna / ID: 2 / Name.synonym: tRNA / Details: co-purified with the ribosomal subunits / Classification: TRANSFER / Structure: DOUBLE HELIX / Synthetic?: No |
|---|---|
| Source (natural) | Organism:    Methanocaldococcus jannaschii (archaea) Methanocaldococcus jannaschii (archaea) |
| Molecular weight | Theoretical: 25 KDa |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 100 mM NH4Cl, 30 mM MgCl2, 20 mM HEPES-KOH, 6 mM beta-mercaptoethanol, 0.1% DDM |
| Grid | Details: Quantifoil 400 mesh 2/1 Cu grids, air glow discharged, and 400 mesh Cu grids with a thin continuous carbon foil |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 77 K / Instrument: FEI VITROBOT MARK III / Method: Blot 1-2 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 51000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 50000 Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Oxford holder / Specimen holder model: GATAN LIQUID NITROGEN |
| Temperature | Average: 93 K |
| Alignment procedure | Legacy - Astigmatism: corrected at 175,000 times magnification |
| Details | Low dose imaging, data collected manually |
| Date | Apr 10, 2008 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Number real images: 217 / Average electron dose: 20 e/Å2 / Details: Zeiss SCAI scanner was also used. / Od range: 1 / Bits/pixel: 16 |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: per micrograph |
|---|---|
| Final two d classification | Number classes: 3800 |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 9.0 Å / Resolution method: OTHER / Software - Name: EMAN1, EMAN2 Details: Final map calculated as an average of 6 different refinements in EMAN2 with different parameters. Number images used: 37000 |
| Details | Particles were selected with e2boxer and CTF-corrected with EMAN2. Final maps were obtained from 6 different refinement conditions, aligned in Chimera, and averaged to produce the final map. |
-Atomic model buiding 1
| Initial model | PDB ID:  3j21 |
|---|---|
| Software | Name: Chimera, MDFF |
| Details | Docked with Chimera and fit with MDFF. Chains omitted: 1('el' 1-77), 6(L83-1), 4(L83-2), c(L33e). Chains truncated: a(L31e) 78-85, W(L29) 67-72, C(L3) 103-125. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-4v4n: |
-Atomic model buiding 2
| Initial model | PDB ID:  3j2l |
|---|---|
| Software | Name: Chimera, MDFF |
| Details | Regions omitted: rRNA chain 1 [1-6, 3047-3049 5' and 3' ends 23S], 150-163, 750-756, 1311-1321, 2904-2942 (ES). |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-4v4n: |
-Atomic model buiding 3
| Initial model | PDB ID:  3j20 |
|---|---|
| Software | Name: Chimera, MDFF |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-4v4n: |
 Movie
Movie Controller
Controller













 Z
Z Y
Y X
X