+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of TRPM7 in MSP2N2 nanodisc in apo state | ||||||||||||||||||
 Map data Map data | Cryo-EM structure of TRPM7 in MSP2N2 nanodisc in apo state | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationintracellular magnesium ion homeostasis / calcium-dependent cell-matrix adhesion / varicosity /  TRP channels / actomyosin structure organization / TRP channels / actomyosin structure organization /  myosin binding / monoatomic cation transmembrane transport / necroptotic process / monoatomic cation channel activity / ruffle ...intracellular magnesium ion homeostasis / calcium-dependent cell-matrix adhesion / varicosity / myosin binding / monoatomic cation transmembrane transport / necroptotic process / monoatomic cation channel activity / ruffle ...intracellular magnesium ion homeostasis / calcium-dependent cell-matrix adhesion / varicosity /  TRP channels / actomyosin structure organization / TRP channels / actomyosin structure organization /  myosin binding / monoatomic cation transmembrane transport / necroptotic process / monoatomic cation channel activity / ruffle / protein tetramerization / myosin binding / monoatomic cation transmembrane transport / necroptotic process / monoatomic cation channel activity / ruffle / protein tetramerization /  calcium channel activity / calcium channel activity /  memory / synaptic vesicle membrane / calcium ion transport / memory / synaptic vesicle membrane / calcium ion transport /  actin binding / actin binding /  kinase activity / kinase activity /  non-specific serine/threonine protein kinase / non-specific serine/threonine protein kinase /  protein kinase activity / positive regulation of apoptotic process / protein kinase activity / positive regulation of apoptotic process /  phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / neuronal cell body / phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / neuronal cell body /  ATP binding / ATP binding /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) | ||||||||||||||||||
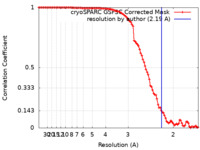
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.19 Å cryo EM / Resolution: 2.19 Å | ||||||||||||||||||
 Authors Authors | Nadezhdin KD / Neuberger A / Sobolevsky AI | ||||||||||||||||||
| Funding support |  United States, United States,  Germany, 5 items Germany, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural mechanisms of TRPM7 activation and inhibition. Authors: Kirill D Nadezhdin / Leonor Correia / Chamali Narangoda / Dhilon S Patel / Arthur Neuberger / Thomas Gudermann / Maria G Kurnikova / Vladimir Chubanov / Alexander I Sobolevsky /   Abstract: The transient receptor potential channel TRPM7 is a master regulator of the organismal balance of divalent cations that plays an essential role in embryonic development, immune responses, cell ...The transient receptor potential channel TRPM7 is a master regulator of the organismal balance of divalent cations that plays an essential role in embryonic development, immune responses, cell mobility, proliferation, and differentiation. TRPM7 is implicated in neuronal and cardiovascular disorders, tumor progression and has emerged as a new drug target. Here we use cryo-EM, functional analysis, and molecular dynamics simulations to uncover two distinct structural mechanisms of TRPM7 activation by a gain-of-function mutation and by the agonist naltriben, which show different conformational dynamics and domain involvement. We identify a binding site for highly potent and selective inhibitors and show that they act by stabilizing the TRPM7 closed state. The discovered structural mechanisms provide foundations for understanding the molecular basis of TRPM7 channelopathies and drug development. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40496.map.gz emd_40496.map.gz | 97.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40496-v30.xml emd-40496-v30.xml emd-40496.xml emd-40496.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40496_fsc.xml emd_40496_fsc.xml | 9.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_40496.png emd_40496.png | 83 KB | ||
| Others |  emd_40496_half_map_1.map.gz emd_40496_half_map_1.map.gz emd_40496_half_map_2.map.gz emd_40496_half_map_2.map.gz | 95.4 MB 95.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40496 http://ftp.pdbj.org/pub/emdb/structures/EMD-40496 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40496 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40496 | HTTPS FTP |
-Related structure data
| Related structure data |  8si2MC  8si3C  8si4C  8si5C  8si6C  8si7C  8si8C  8siaC  8sibC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40496.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40496.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of TRPM7 in MSP2N2 nanodisc in apo state | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 1
| File | emd_40496_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 2
| File | emd_40496_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : sample 1
| Entire | Name: sample 1 |
|---|---|
| Components |
|
-Supramolecule #1: sample 1
| Supramolecule | Name: sample 1 / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 700 KDa |
-Macromolecule #1: Transient receptor potential cation channel subfamily M member 7
| Macromolecule | Name: Transient receptor potential cation channel subfamily M member 7 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number:  non-specific serine/threonine protein kinase non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 146.888875 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: SQKSWIESTL TKRECVYIIP SSKDPHRCLP GCQICQQLVR CFCGRLVKQH ACFTASLAMK YSDVKLGEHF NQAIEEWSVE KHTEQSPTD AYGVINFQGG SHSYRAKYVR LSYDTKPEII LQLLLKEWQM ELPKLVISVH GGMQKFELHP RIKQLLGKGL I KAAVTTGA ...String: SQKSWIESTL TKRECVYIIP SSKDPHRCLP GCQICQQLVR CFCGRLVKQH ACFTASLAMK YSDVKLGEHF NQAIEEWSVE KHTEQSPTD AYGVINFQGG SHSYRAKYVR LSYDTKPEII LQLLLKEWQM ELPKLVISVH GGMQKFELHP RIKQLLGKGL I KAAVTTGA WILTGGVNTG VAKHVGDALK EHASRSSRKI CTIGIAPWGV IENRNDLVGR DVVAPYQTLL NPLSKLNVLN NL HSHFILV DDGTVGKYGA EVRLRRELEK TINQQRIHAR IGQGVPVVAL IFEGGPNVIL TVLEYLQESP PVPVVVCEGT GRA ADLLAY IHKQTEEGGN LPDAAEPDII STIKKTFNFG QSEAVHLFQT MMECMKKKEL ITVFHIGSED HQDIDVAILT ALLK GTNAS AFDQLILTLA WDRVDIAKNH VFVYGQQWLV GSLEQAMLDA LVMDRVSFVK LLIENGVSMH KFLTIPRLEE LYNTK QGPT NPMLFHLIRD VKQGNLPPGY KITLIDIGLV IEYLMGGTYR CTYTRKRFRL IYNSLGGNNR RSGRNTSSST PQLRKS HET FGNRADKKEK MRHNHFIKTA QPYRPKMDAS MEEGKKKRTK DEIVDIDDPE TKRFPYPLNE LLIWACLMKR QVMARFL WQ HGEESMAKAL VACKIYRSMA YEAKQSDLVD DTSEELKQYS NDFGQLAVEL LEQSFRQDET MAMKLLTYEL KNWSNSTC L KLAVSSRLRP FVAHTCTQML LSDMWMGRLN MRKNSWYKVI LSILVPPAIL MLEYKTKAEM SHIPQSQDAH QMTMEDSEN NFHNITEEIP MEVFKEVKIL DSSDGKNEME IHIKSKKLPI TRKFYAFYHA PIVKFWFNTL AYLGFLMLYT FVVLVKMEQL PSVQEWIVI AYIFTYAIEK VREVFMSEAG KISQKIKVWF SDYFNVSDTI AIISFFVGFG LRFGAKWNYI NAYDNHVFVA G RLIYCLNI IFWYVRLLDF LAVNQQAGPY VMMIGKMVAN MFYIVVIMAL VLLSFGVPRK AILYPHEEPS WSLAKDIVFH PY WMIFGEV YAYEIDVCAN DSTLPTICGP GTWLTPFLQA VYLFVQYIIM VNLLIAFFNN VYLQVKAISN IVWKYQRYHF IMA YHEKPV LPPPLIILSH IVSLFCCVCK RRKKDKTSDG PKLFLTEEDQ KKLHDFEEQC VEMYFDEKDD KFNSGSEERI RVTF ERVEQ MSIQIKEVGD RVNYIKRSLQ SLDSQIGHLQ DLSALTVDTL KTLTAQKASE ASKVHNEITR ELSISKHLAQ NLID |
-Macromolecule #2: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 2 / Number of copies: 64 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Macromolecule #3: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 3 / Number of copies: 4 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #4: 2-[2-[(1~{S},2~{S},4~{S},5'~{R},6~{R},7~{S},8~{R},9~{S},12~{S},13...
| Macromolecule | Name: 2-[2-[(1~{S},2~{S},4~{S},5'~{R},6~{R},7~{S},8~{R},9~{S},12~{S},13~{R},16~{S})-5',7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icos-18-ene-6,2'-oxane]-16-yl]oxyethyl]propane-1,3-diol type: ligand / ID: 4 / Number of copies: 4 / Formula: DU0 |
|---|---|
| Molecular weight | Theoretical: 516.752 Da |
| Chemical component information |  ChemComp-DU0: |
-Macromolecule #5: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 5 / Number of copies: 2 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 168 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.2 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 25 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||
| Details | mouse TRPM7 |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 5087 / Average exposure time: 2.5 sec. / Average electron dose: 58.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-8si2: |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X