+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
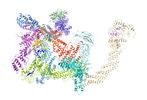
| Title | Cryo-EM Structure of the KBTBD2-CRL3~N8(removed)-CSN complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  ligase / ligase /  complex complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator /  COP9 signalosome assembly / trophectodermal cell proliferation / COP9 signalosome assembly / trophectodermal cell proliferation /  macrophage migration inhibitory factor binding / liver morphogenesis / positive regulation of mitotic cell cycle phase transition / trophectodermal cellular morphogenesis / POZ domain binding / regulation of IRE1-mediated unfolded protein response / exosomal secretion ...regulation of DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator / macrophage migration inhibitory factor binding / liver morphogenesis / positive regulation of mitotic cell cycle phase transition / trophectodermal cellular morphogenesis / POZ domain binding / regulation of IRE1-mediated unfolded protein response / exosomal secretion ...regulation of DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator /  COP9 signalosome assembly / trophectodermal cell proliferation / COP9 signalosome assembly / trophectodermal cell proliferation /  macrophage migration inhibitory factor binding / liver morphogenesis / positive regulation of mitotic cell cycle phase transition / trophectodermal cellular morphogenesis / POZ domain binding / regulation of IRE1-mediated unfolded protein response / exosomal secretion / nuclear protein quality control by the ubiquitin-proteasome system / deNEDDylase activity / GTPase inhibitor activity / macrophage migration inhibitory factor binding / liver morphogenesis / positive regulation of mitotic cell cycle phase transition / trophectodermal cellular morphogenesis / POZ domain binding / regulation of IRE1-mediated unfolded protein response / exosomal secretion / nuclear protein quality control by the ubiquitin-proteasome system / deNEDDylase activity / GTPase inhibitor activity /  regulation protein catabolic process at postsynapse / polar microtubule / regulation of protein neddylation / eukaryotic translation initiation factor 3 complex / protein deneddylation / COPII vesicle coating / anaphase-promoting complex-dependent catabolic process / positive regulation of mitotic metaphase/anaphase transition / cullin-RING-type E3 NEDD8 transferase / stem cell division / cellular response to chemical stress / NEDD8 transferase activity / RHOBTB3 ATPase cycle / cullin-RING ubiquitin ligase complex / embryonic cleavage / cell projection organization / regulation protein catabolic process at postsynapse / polar microtubule / regulation of protein neddylation / eukaryotic translation initiation factor 3 complex / protein deneddylation / COPII vesicle coating / anaphase-promoting complex-dependent catabolic process / positive regulation of mitotic metaphase/anaphase transition / cullin-RING-type E3 NEDD8 transferase / stem cell division / cellular response to chemical stress / NEDD8 transferase activity / RHOBTB3 ATPase cycle / cullin-RING ubiquitin ligase complex / embryonic cleavage / cell projection organization /  COP9 signalosome / Cul7-RING ubiquitin ligase complex / metal-dependent deubiquitinase activity / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / activation of NF-kappaB-inducing kinase activity / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / positive regulation of protein autoubiquitination / protein neddylation / COP9 signalosome / Cul7-RING ubiquitin ligase complex / metal-dependent deubiquitinase activity / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / activation of NF-kappaB-inducing kinase activity / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / positive regulation of protein autoubiquitination / protein neddylation /  Hydrolases; Acting on peptide bonds (peptidases) / Notch binding / NEDD8 ligase activity / Cul5-RING ubiquitin ligase complex / RHOBTB1 GTPase cycle / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / fibroblast apoptotic process / ubiquitin-ubiquitin ligase activity / Cul4A-RING E3 ubiquitin ligase complex / negative regulation of Rho protein signal transduction / Cul2-RING ubiquitin ligase complex / Hydrolases; Acting on peptide bonds (peptidases) / Notch binding / NEDD8 ligase activity / Cul5-RING ubiquitin ligase complex / RHOBTB1 GTPase cycle / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / fibroblast apoptotic process / ubiquitin-ubiquitin ligase activity / Cul4A-RING E3 ubiquitin ligase complex / negative regulation of Rho protein signal transduction / Cul2-RING ubiquitin ligase complex /  SCF ubiquitin ligase complex / inner cell mass cell proliferation / negative regulation of type I interferon production / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / mitotic metaphase chromosome alignment / Cul3-RING ubiquitin ligase complex / SCF ubiquitin ligase complex / inner cell mass cell proliferation / negative regulation of type I interferon production / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / mitotic metaphase chromosome alignment / Cul3-RING ubiquitin ligase complex /  stress fiber assembly / protein deubiquitination / Prolactin receptor signaling / positive regulation of cytokinesis / skeletal muscle cell differentiation / protein monoubiquitination / cullin family protein binding / regulation of JNK cascade / response to light stimulus / stress fiber assembly / protein deubiquitination / Prolactin receptor signaling / positive regulation of cytokinesis / skeletal muscle cell differentiation / protein monoubiquitination / cullin family protein binding / regulation of JNK cascade / response to light stimulus /  gastrulation / protein K48-linked ubiquitination / sperm flagellum / endoplasmic reticulum to Golgi vesicle-mediated transport / protein autoubiquitination / RHOBTB2 GTPase cycle / Nuclear events stimulated by ALK signaling in cancer / JNK cascade / positive regulation of TORC1 signaling / gastrulation / protein K48-linked ubiquitination / sperm flagellum / endoplasmic reticulum to Golgi vesicle-mediated transport / protein autoubiquitination / RHOBTB2 GTPase cycle / Nuclear events stimulated by ALK signaling in cancer / JNK cascade / positive regulation of TORC1 signaling /  translation initiation factor activity / translation initiation factor activity /  Regulation of BACH1 activity / Regulation of BACH1 activity /  cyclin binding / cyclin binding /  T cell activation / T cell activation /  post-translational protein modification / intrinsic apoptotic signaling pathway / phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of protein ubiquitination / integrin-mediated signaling pathway / Degradation of DVL / Recognition of DNA damage by PCNA-containing replication complex / cellular response to amino acid stimulus / Degradation of GLI1 by the proteasome / Negative regulation of NOTCH4 signaling / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Vif-mediated degradation of APOBEC3G / Hedgehog 'on' state / G1/S transition of mitotic cell cycle / DNA Damage Recognition in GG-NER / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / protein destabilization / response to insulin / RING-type E3 ubiquitin transferase / Degradation of beta-catenin by the destruction complex / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha post-translational protein modification / intrinsic apoptotic signaling pathway / phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of protein ubiquitination / integrin-mediated signaling pathway / Degradation of DVL / Recognition of DNA damage by PCNA-containing replication complex / cellular response to amino acid stimulus / Degradation of GLI1 by the proteasome / Negative regulation of NOTCH4 signaling / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Vif-mediated degradation of APOBEC3G / Hedgehog 'on' state / G1/S transition of mitotic cell cycle / DNA Damage Recognition in GG-NER / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / protein destabilization / response to insulin / RING-type E3 ubiquitin transferase / Degradation of beta-catenin by the destruction complex / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor AlphaSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 7.51 Å cryo EM / Resolution: 7.51 Å | |||||||||
 Authors Authors | Hu Y / Mao Q / Chen Z / Sun L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Dynamic molecular architecture and substrate recruitment of cullin3-RING E3 ligase CRL3. Authors: Yuxia Hu / Zhao Zhang / Qiyu Mao / Xiang Zhang / Aihua Hao / Yu Xun / Yeda Wang / Lin Han / Wuqiang Zhan / Qianying Liu / Yue Yin / Chao Peng / Eva Marie Y Moresco / Zhenguo Chen / Bruce Beutler / Lei Sun /   Abstract: Phosphatidylinositol 3-kinase α, a heterodimer of catalytic p110α and one of five regulatory subunits, mediates insulin- and insulin like growth factor-signaling and, frequently, oncogenesis. ...Phosphatidylinositol 3-kinase α, a heterodimer of catalytic p110α and one of five regulatory subunits, mediates insulin- and insulin like growth factor-signaling and, frequently, oncogenesis. Cellular levels of the regulatory p85α subunit are tightly controlled by regulated proteasomal degradation. In adipose tissue and growth plates, failure of K48-linked p85α ubiquitination causes diabetes, lipodystrophy and dwarfism in mice, as in humans with SHORT syndrome. Here we elucidated the structures of the key ubiquitin ligase complexes regulating p85α availability. Specificity is provided by the substrate receptor KBTBD2, which recruits p85α to the cullin3-RING E3 ubiquitin ligase (CRL3). CRL3 forms multimers, which disassemble into dimers upon substrate binding (CRL3-p85α) and/or neddylation by the activator NEDD8 (CRL3~N8), leading to p85α ubiquitination and degradation. Deactivation involves dissociation of NEDD8 mediated by the COP9 signalosome and displacement of KBTBD2 by the inhibitor CAND1. The hereby identified structural basis of p85α regulation opens the way to better understanding disturbances of glucose regulation, growth and cancer. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34462.map.gz emd_34462.map.gz | 34.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34462-v30.xml emd-34462-v30.xml emd-34462.xml emd-34462.xml | 31.1 KB 31.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34462.png emd_34462.png | 99.3 KB | ||
| Filedesc metadata |  emd-34462.cif.gz emd-34462.cif.gz | 9.1 KB | ||
| Others |  emd_34462_half_map_1.map.gz emd_34462_half_map_1.map.gz emd_34462_half_map_2.map.gz emd_34462_half_map_2.map.gz | 29.6 MB 29.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34462 http://ftp.pdbj.org/pub/emdb/structures/EMD-34462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34462 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34462 | HTTPS FTP |
-Related structure data
| Related structure data |  8h3aMC  8gq6C  8h33C  8h34C  8h35C  8h36C  8h37C  8h38C  8h3fC  8h3qC  8h3rC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34462.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34462.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 2.088 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34462_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_34462_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Cryo-EM Structure of the KBTBD2-CRL3~N8-CSN complex
+Supramolecule #1: Cryo-EM Structure of the KBTBD2-CRL3~N8-CSN complex
+Supramolecule #2: CSN
+Supramolecule #3: KBTBD2, Cullin-3, Rbx1
+Macromolecule #1: COP9 signalosome complex subunit 5
+Macromolecule #2: COP9 signalosome complex subunit 1
+Macromolecule #3: COP9 signalosome complex subunit 2
+Macromolecule #4: COP9 signalosome complex subunit 3
+Macromolecule #5: COP9 signalosome complex subunit 4
+Macromolecule #6: COP9 signalosome complex subunit 6
+Macromolecule #7: COP9 signalosome complex subunit 7b
+Macromolecule #8: COP9 signalosome complex subunit 8
+Macromolecule #9: Kelch repeat and BTB domain-containing protein 2
+Macromolecule #10: Cullin-3
+Macromolecule #11: E3 ubiquitin-protein ligase RBX1
+Macromolecule #12: ZINC ION
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 53.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: Details: 6r7f |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 7.51 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0) / Number images used: 55391 |
 Movie
Movie Controller
Controller

































 Z
Z Y
Y X
X