Entry Database : EMDB / ID : EMD-32735Title Structure of Human IGF1/IGFBP3/ALS Ternary Complex Complex : Ternary complex of IGF1/IGFBP3/ALSProtein or peptide : Insulin-like growth factor-binding protein complex acid labile subunitProtein or peptide : Insulin-like growth factor-binding protein 3Protein or peptide : Isoform 3 of Insulin-like growth factor ILigand : 2-acetamido-2-deoxy-beta-D-glucopyranoseFunction / homology Function Domain/homology Component
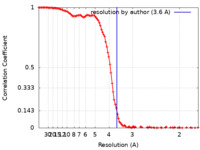
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / Resolution : 3.6 Å Kim H / Fu Y / Kim HM Funding support 1 items Organization Grant number Country Other government Institure of Basic Science, IBS-R030-C1
Journal : Nat Commun / Year : 2022Title : Structural basis for assembly and disassembly of the IGF/IGFBP/ALS ternary complexAuthors : Kim H / Fu Y / Hong HJ / Lee SG / Lee DS / Kim HM History Deposition Jan 27, 2022 - Header (metadata) release Aug 10, 2022 - Map release Aug 10, 2022 - Update Aug 10, 2022 - Current status Aug 10, 2022 Processing site : PDBj / Status : Released
Show all Show less
 Open data
Open data Basic information
Basic information
 Map data
Map data Sample
Sample Function and homology information
Function and homology information muscle hypertrophy / negative regulation of oocyte development / positive regulation of trophectodermal cell proliferation / insulin-like growth factor binding protein complex / insulin-like growth factor ternary complex / proteoglycan biosynthetic process / negative regulation of smooth muscle cell migration ...protein tyrosine phosphatase activator activity / regulation of insulin-like growth factor receptor signaling pathway / glycolate metabolic process /
muscle hypertrophy / negative regulation of oocyte development / positive regulation of trophectodermal cell proliferation / insulin-like growth factor binding protein complex / insulin-like growth factor ternary complex / proteoglycan biosynthetic process / negative regulation of smooth muscle cell migration ...protein tyrosine phosphatase activator activity / regulation of insulin-like growth factor receptor signaling pathway / glycolate metabolic process /  muscle hypertrophy / negative regulation of oocyte development / positive regulation of trophectodermal cell proliferation / insulin-like growth factor binding protein complex / insulin-like growth factor ternary complex / proteoglycan biosynthetic process / negative regulation of smooth muscle cell migration / positive regulation of glycoprotein biosynthetic process / myotube cell development / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration / negative regulation of neuroinflammatory response / negative regulation of vascular associated smooth muscle cell apoptotic process / bone mineralization involved in bone maturation / Signaling by Type 1 Insulin-like Growth Factor 1 Receptor (IGF1R) / positive regulation of cell growth involved in cardiac muscle cell development / IRS-related events triggered by IGF1R /
muscle hypertrophy / negative regulation of oocyte development / positive regulation of trophectodermal cell proliferation / insulin-like growth factor binding protein complex / insulin-like growth factor ternary complex / proteoglycan biosynthetic process / negative regulation of smooth muscle cell migration / positive regulation of glycoprotein biosynthetic process / myotube cell development / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration / negative regulation of neuroinflammatory response / negative regulation of vascular associated smooth muscle cell apoptotic process / bone mineralization involved in bone maturation / Signaling by Type 1 Insulin-like Growth Factor 1 Receptor (IGF1R) / positive regulation of cell growth involved in cardiac muscle cell development / IRS-related events triggered by IGF1R /  insulin-like growth factor binding / exocytic vesicle / cell activation / positive regulation of calcineurin-NFAT signaling cascade /
insulin-like growth factor binding / exocytic vesicle / cell activation / positive regulation of calcineurin-NFAT signaling cascade /  insulin-like growth factor II binding / type B pancreatic cell proliferation / positive regulation of transcription regulatory region DNA binding /
insulin-like growth factor II binding / type B pancreatic cell proliferation / positive regulation of transcription regulatory region DNA binding /  insulin-like growth factor I binding / alphav-beta3 integrin-IGF-1-IGF1R complex / positive regulation of Ras protein signal transduction / myoblast differentiation / myoblast proliferation / positive regulation of insulin-like growth factor receptor signaling pathway / muscle organ development / TP53 Regulates Transcription of Death Receptors and Ligands / negative regulation of interleukin-1 beta production / positive regulation of activated T cell proliferation / positive regulation of cardiac muscle hypertrophy / positive regulation of smooth muscle cell migration / negative regulation of release of cytochrome c from mitochondria / negative regulation of amyloid-beta formation / negative regulation of smooth muscle cell apoptotic process /
insulin-like growth factor I binding / alphav-beta3 integrin-IGF-1-IGF1R complex / positive regulation of Ras protein signal transduction / myoblast differentiation / myoblast proliferation / positive regulation of insulin-like growth factor receptor signaling pathway / muscle organ development / TP53 Regulates Transcription of Death Receptors and Ligands / negative regulation of interleukin-1 beta production / positive regulation of activated T cell proliferation / positive regulation of cardiac muscle hypertrophy / positive regulation of smooth muscle cell migration / negative regulation of release of cytochrome c from mitochondria / negative regulation of amyloid-beta formation / negative regulation of smooth muscle cell apoptotic process /  fibronectin binding / negative regulation of tumor necrosis factor production / regulation of glucose metabolic process / Synthesis, secretion, and deacylation of Ghrelin /
fibronectin binding / negative regulation of tumor necrosis factor production / regulation of glucose metabolic process / Synthesis, secretion, and deacylation of Ghrelin /  epithelial to mesenchymal transition / positive regulation of glycogen biosynthetic process / positive regulation of myoblast differentiation / positive regulation of osteoblast differentiation / positive regulation of DNA binding / SHC-related events triggered by IGF1R / negative regulation of signal transduction / positive regulation of tyrosine phosphorylation of STAT protein / positive regulation of vascular associated smooth muscle cell proliferation /
epithelial to mesenchymal transition / positive regulation of glycogen biosynthetic process / positive regulation of myoblast differentiation / positive regulation of osteoblast differentiation / positive regulation of DNA binding / SHC-related events triggered by IGF1R / negative regulation of signal transduction / positive regulation of tyrosine phosphorylation of STAT protein / positive regulation of vascular associated smooth muscle cell proliferation /  insulin-like growth factor receptor binding / activation of protein kinase B activity / positive regulation of glycolytic process /
insulin-like growth factor receptor binding / activation of protein kinase B activity / positive regulation of glycolytic process /  extracellular matrix / positive regulation of mitotic nuclear division / insulin-like growth factor receptor signaling pathway / negative regulation of protein phosphorylation / platelet alpha granule lumen /
extracellular matrix / positive regulation of mitotic nuclear division / insulin-like growth factor receptor signaling pathway / negative regulation of protein phosphorylation / platelet alpha granule lumen /  skeletal system development / positive regulation of epithelial cell proliferation /
skeletal system development / positive regulation of epithelial cell proliferation /  regulation of cell growth / positive regulation of protein secretion / negative regulation of extrinsic apoptotic signaling pathway / negative regulation of smooth muscle cell proliferation / positive regulation of glucose import /
regulation of cell growth / positive regulation of protein secretion / negative regulation of extrinsic apoptotic signaling pathway / negative regulation of smooth muscle cell proliferation / positive regulation of glucose import /  Post-translational protein phosphorylation / positive regulation of smooth muscle cell proliferation /
Post-translational protein phosphorylation / positive regulation of smooth muscle cell proliferation /  regulation of protein phosphorylation /
regulation of protein phosphorylation /  growth factor activity /
growth factor activity /  wound healing /
wound healing /  insulin receptor binding /
insulin receptor binding /  hormone activity / osteoblast differentiation / cellular response to amyloid-beta / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / positive regulation of fibroblast proliferation /
hormone activity / osteoblast differentiation / cellular response to amyloid-beta / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / positive regulation of fibroblast proliferation /  MAPK cascade / positive regulation of peptidyl-tyrosine phosphorylation /
MAPK cascade / positive regulation of peptidyl-tyrosine phosphorylation /  integrin binding / Platelet degranulation / response to heat /
integrin binding / Platelet degranulation / response to heat /  regulation of gene expression / Ras protein signal transduction / cell population proliferation / positive regulation of MAPK cascade / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of ERK1 and ERK2 cascade / protein stabilization /
regulation of gene expression / Ras protein signal transduction / cell population proliferation / positive regulation of MAPK cascade / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of ERK1 and ERK2 cascade / protein stabilization /  cell adhesion / positive regulation of cell migration / positive regulation of apoptotic process / negative regulation of cell population proliferation /
cell adhesion / positive regulation of cell migration / positive regulation of apoptotic process / negative regulation of cell population proliferation /  endoplasmic reticulum lumen /
endoplasmic reticulum lumen /  protein phosphorylation / negative regulation of gene expression / apoptotic process
protein phosphorylation / negative regulation of gene expression / apoptotic process
 Homo sapiens (human)
Homo sapiens (human) single particle reconstruction /
single particle reconstruction /  cryo EM / Resolution: 3.6 Å
cryo EM / Resolution: 3.6 Å  Authors
Authors Citation
Citation Journal: Nat Commun / Year: 2022
Journal: Nat Commun / Year: 2022 Structure visualization
Structure visualization Downloads & links
Downloads & links emd_32735.map.gz
emd_32735.map.gz EMDB map data format
EMDB map data format emd-32735-v30.xml
emd-32735-v30.xml emd-32735.xml
emd-32735.xml EMDB header
EMDB header emd_32735_fsc.xml
emd_32735_fsc.xml FSC data file
FSC data file emd_32735.png
emd_32735.png emd_32735_msk_1.map
emd_32735_msk_1.map Mask map
Mask map emd_32735_half_map_1.map.gz
emd_32735_half_map_1.map.gz emd_32735_half_map_2.map.gz
emd_32735_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-32735
http://ftp.pdbj.org/pub/emdb/structures/EMD-32735 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32735
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32735
 F&H Search
F&H Search Links
Links EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource Map
Map Download / File: emd_32735.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
Download / File: emd_32735.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) emd_32735_msk_1.map
emd_32735_msk_1.map Sample components
Sample components
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human)
Homo sapiens (human)
 cryo EM
cryo EM Processing
Processing single particle reconstruction
single particle reconstruction Sample preparation
Sample preparation Electron microscopy
Electron microscopy FIELD EMISSION GUN
FIELD EMISSION GUN Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.7 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000
Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.7 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000 
 Movie
Movie Controller
Controller

























 Z
Z Y
Y X
X