+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3052 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure and mechanism of the Rubisco assembly chaperone Raf1 | |||||||||
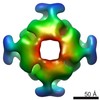
 Map data Map data | Negative stain 3D reconstruction of crosslinked S. elongatus RbcL8 and Syn7942-Raf1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Rubisco / Chaperone / Rubisco / Chaperone /  Raf1 / assembly Raf1 / assembly | |||||||||
| Function / homology | Chaperonin-like RbcX Function and homology information Function and homology information | |||||||||
| Biological species |   Synechococcus elongatus PCC 6301 (bacteria) Synechococcus elongatus PCC 6301 (bacteria) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 21.4 Å negative staining / Resolution: 21.4 Å | |||||||||
 Authors Authors | Hauser A / Bhat JY / Milicic G / Wendler P / Hartl FU / Bracher A / Hayer-Hartl M | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2015 Journal: Nat Struct Mol Biol / Year: 2015Title: Structure and mechanism of the Rubisco-assembly chaperone Raf1. Authors: Thomas Hauser / Javaid Y Bhat / Goran Miličić / Petra Wendler / F Ulrich Hartl / Andreas Bracher / Manajit Hayer-Hartl /  Abstract: Biogenesis of the photosynthetic enzyme Rubisco, a complex of eight large (RbcL) and eight small (RbcS) subunits, requires assembly chaperones. Here we analyzed the role of Rubisco accumulation ...Biogenesis of the photosynthetic enzyme Rubisco, a complex of eight large (RbcL) and eight small (RbcS) subunits, requires assembly chaperones. Here we analyzed the role of Rubisco accumulation factor1 (Raf1), a dimer of ∼40-kDa subunits. We find that Raf1 from Synechococcus elongatus acts downstream of chaperonin-assisted RbcL folding by stabilizing RbcL antiparallel dimers for assembly into RbcL8 complexes with four Raf1 dimers bound. Raf1 displacement by RbcS results in holoenzyme formation. Crystal structures show that Raf1 from Arabidopsis thaliana consists of a β-sheet dimerization domain and a flexibly linked α-helical domain. Chemical cross-linking and EM reconstruction indicate that the β-domains bind along the equator of each RbcL2 unit, and the α-helical domains embrace the top and bottom edges of RbcL2. Raf1 fulfills a role similar to that of the assembly chaperone RbcX, thus suggesting that functionally redundant factors ensure efficient Rubisco biogenesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3052.map.gz emd_3052.map.gz | 17.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3052-v30.xml emd-3052-v30.xml emd-3052.xml emd-3052.xml | 10.2 KB 10.2 KB | Display Display |  EMDB header EMDB header |
| Images |  EMDB3052.png EMDB3052.png | 353.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3052 http://ftp.pdbj.org/pub/emdb/structures/EMD-3052 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3052 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3052 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3052.map.gz / Format: CCP4 / Size: 26.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3052.map.gz / Format: CCP4 / Size: 26.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative stain 3D reconstruction of crosslinked S. elongatus RbcL8 and Syn7942-Raf1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Synechococcus elongatus RbcL/Raf1
| Entire | Name: Synechococcus elongatus RbcL/Raf1 |
|---|---|
| Components |
|
-Supramolecule #1000: Synechococcus elongatus RbcL/Raf1
| Supramolecule | Name: Synechococcus elongatus RbcL/Raf1 / type: sample / ID: 1000 / Oligomeric state: Four Raf1 dimers bind one RbcL octamer / Number unique components: 2 |
|---|---|
| Molecular weight | Experimental: 740 KDa / Theoretical: 740 KDa / Method: native Mass Spectrometry |
-Macromolecule #1: Ribulose-1,5-bisphosphate carboxylase oxygenase
| Macromolecule | Name: Ribulose-1,5-bisphosphate carboxylase oxygenase / type: protein_or_peptide / ID: 1 / Name.synonym: RuBisCO Details: complex is cross linked with bifunctional crosslinker disuccinimidylsuberate (DSS) Number of copies: 8 / Oligomeric state: Octamer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Synechococcus elongatus PCC 6301 (bacteria) / Organelle: chloroplast Synechococcus elongatus PCC 6301 (bacteria) / Organelle: chloroplast |
| Molecular weight | Experimental: 42 KDa / Theoretical: 42 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | InterPro: Chaperonin-like RbcX |
-Macromolecule #2: Rubisco accumulation factor 1
| Macromolecule | Name: Rubisco accumulation factor 1 / type: protein_or_peptide / ID: 2 / Name.synonym: Raf1 Details: complex is cross linked with bifunctional crosslinker disuccinimidylsuberate (DSS) Number of copies: 8 / Oligomeric state: dimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Synechococcus elongatus PCC 6301 (bacteria) / Organelle: Chloroplast Synechococcus elongatus PCC 6301 (bacteria) / Organelle: Chloroplast |
| Molecular weight | Theoretical: 40 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) / Recombinant plasmid: pHUE Escherichia coli BL21(DE3) (bacteria) / Recombinant plasmid: pHUE |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.05 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 20mM MOPS-KOH, 100mM KCl, 5mM Mg acetate, 5mM DTT |
| Staining | Type: NEGATIVE / Details: Grids were stained with 2% uranyl acetate for 30s |
| Grid | Details: Plasma cleaned carbon coated grids (Quantifoil) |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Electron beam | Acceleration voltage: 160 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 69500 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.3 µm / Nominal magnification: 50000 Bright-field microscopy / Cs: 2 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.3 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: negative stain / Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Details | low dose |
| Date | Apr 24, 2015 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F415 (4k x 4k) / Digitization - Sampling interval: 15 µm / Number real images: 49 / Average electron dose: 20 e/Å2 |
- Image processing
Image processing
| CTF correction | Details: Relion |
|---|---|
| Final reconstruction | Applied symmetry - Point group: D4 (2x4 fold dihedral ) / Resolution.type: BY AUTHOR / Resolution: 21.4 Å / Resolution method: OTHER / Software - Name: MRC, RELION, 1.3 / Number images used: 5183 ) / Resolution.type: BY AUTHOR / Resolution: 21.4 Å / Resolution method: OTHER / Software - Name: MRC, RELION, 1.3 / Number images used: 5183 |
| Details | particles were selected manually |
 Movie
Movie Controller
Controller