[English] 日本語
 Yorodumi
Yorodumi- EMDB-29663: CryoEM structure of cytoplasmic GAPDH under 24h Oxidative Stress -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of cytoplasmic GAPDH under 24h Oxidative Stress | |||||||||
 Map data Map data | GAPDH, cyto, oxidative stress 24h, overall, d2 Glyceraldehyde 3-phosphate dehydrogenase Glyceraldehyde 3-phosphate dehydrogenase | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  GAPDH / GAPDH /  energy / energy /  cytosolic protein / cytosolic protein /  TRANSFERASE TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology information Transferases; Transferring nitrogenous groups; Transferring other nitrogenous groups / peptidyl-cysteine S-nitrosylase activity / peptidyl-cysteine S-trans-nitrosylation / Transferases; Transferring nitrogenous groups; Transferring other nitrogenous groups / peptidyl-cysteine S-nitrosylase activity / peptidyl-cysteine S-trans-nitrosylation /  glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) / killing by host of symbiont cells / glyceraldehyde-3-phosphate dehydrogenase (NAD+) (phosphorylating) activity / negative regulation of endopeptidase activity / aspartic-type endopeptidase inhibitor activity / glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) / killing by host of symbiont cells / glyceraldehyde-3-phosphate dehydrogenase (NAD+) (phosphorylating) activity / negative regulation of endopeptidase activity / aspartic-type endopeptidase inhibitor activity /  Gluconeogenesis / Gluconeogenesis /  GAIT complex ... GAIT complex ... Transferases; Transferring nitrogenous groups; Transferring other nitrogenous groups / peptidyl-cysteine S-nitrosylase activity / peptidyl-cysteine S-trans-nitrosylation / Transferases; Transferring nitrogenous groups; Transferring other nitrogenous groups / peptidyl-cysteine S-nitrosylase activity / peptidyl-cysteine S-trans-nitrosylation /  glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) / killing by host of symbiont cells / glyceraldehyde-3-phosphate dehydrogenase (NAD+) (phosphorylating) activity / negative regulation of endopeptidase activity / aspartic-type endopeptidase inhibitor activity / glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) / killing by host of symbiont cells / glyceraldehyde-3-phosphate dehydrogenase (NAD+) (phosphorylating) activity / negative regulation of endopeptidase activity / aspartic-type endopeptidase inhibitor activity /  Gluconeogenesis / Gluconeogenesis /  GAIT complex / GAIT complex /  Glycolysis / positive regulation of type I interferon production / Glycolysis / positive regulation of type I interferon production /  regulation of macroautophagy / defense response to fungus / regulation of macroautophagy / defense response to fungus /  lipid droplet / positive regulation of cytokine production / glycolytic process / microtubule cytoskeleton organization / cellular response to type II interferon / glucose metabolic process / NAD binding / microtubule cytoskeleton / disordered domain specific binding / antimicrobial humoral immune response mediated by antimicrobial peptide / lipid droplet / positive regulation of cytokine production / glycolytic process / microtubule cytoskeleton organization / cellular response to type II interferon / glucose metabolic process / NAD binding / microtubule cytoskeleton / disordered domain specific binding / antimicrobial humoral immune response mediated by antimicrobial peptide /  NADP binding / NADP binding /  microtubule binding / neuron apoptotic process / positive regulation of canonical NF-kappaB signal transduction / microtubule binding / neuron apoptotic process / positive regulation of canonical NF-kappaB signal transduction /  nuclear membrane / killing of cells of another organism / vesicle / negative regulation of translation / protein stabilization / nuclear membrane / killing of cells of another organism / vesicle / negative regulation of translation / protein stabilization /  ribonucleoprotein complex / intracellular membrane-bounded organelle / perinuclear region of cytoplasm / extracellular exosome / ribonucleoprotein complex / intracellular membrane-bounded organelle / perinuclear region of cytoplasm / extracellular exosome /  membrane / identical protein binding / membrane / identical protein binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.07 Å cryo EM / Resolution: 2.07 Å | |||||||||
 Authors Authors | Choi WY / Wu H / Cheng YF / Manglik A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Efficient tagging of endogenous proteins in human cell lines for structural studies by single-particle cryo-EM. Authors: Wooyoung Choi / Hao Wu / Klaus Yserentant / Bo Huang / Yifan Cheng /  Abstract: CRISPR/Cas9-based genome engineering has revolutionized our ability to manipulate biological systems, particularly in higher organisms. Here, we designed a set of homology-directed repair donor ...CRISPR/Cas9-based genome engineering has revolutionized our ability to manipulate biological systems, particularly in higher organisms. Here, we designed a set of homology-directed repair donor templates that enable efficient tagging of endogenous proteins with affinity tags by transient transfection and selection of genome-edited cells in various human cell lines. Combined with technological advancements in single-particle cryogenic electron microscopy, this strategy allows efficient structural studies of endogenous proteins captured in their native cellular environment and during different cellular processes. We demonstrated this strategy by tagging six different human proteins in both HEK293T and Jurkat cells. Moreover, analysis of endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in HEK293T cells allowed us to follow its behavior spatially and temporally in response to prolonged oxidative stress, correlating the increased number of oxidation-induced inactive catalytic sites in GAPDH with its translocation from cytosol to nucleus. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29663.map.gz emd_29663.map.gz | 39.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29663-v30.xml emd-29663-v30.xml emd-29663.xml emd-29663.xml | 24.1 KB 24.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29663.png emd_29663.png | 94.7 KB | ||
| Filedesc metadata |  emd-29663.cif.gz emd-29663.cif.gz | 5.4 KB | ||
| Others |  emd_29663_additional_1.map.gz emd_29663_additional_1.map.gz emd_29663_additional_2.map.gz emd_29663_additional_2.map.gz emd_29663_additional_3.map.gz emd_29663_additional_3.map.gz emd_29663_additional_4.map.gz emd_29663_additional_4.map.gz emd_29663_additional_5.map.gz emd_29663_additional_5.map.gz emd_29663_half_map_1.map.gz emd_29663_half_map_1.map.gz emd_29663_half_map_2.map.gz emd_29663_half_map_2.map.gz | 1.2 MB 1.3 MB 1.3 MB 1.3 MB 1.2 MB 39.7 MB 39.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29663 http://ftp.pdbj.org/pub/emdb/structures/EMD-29663 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29663 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29663 | HTTPS FTP |
-Related structure data
| Related structure data |  8g16MC  8g12C  8g13C  8g14C  8g15C  8g17C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29663.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29663.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, overall, d2 | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.835 Å | ||||||||||||||||||||
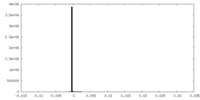
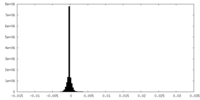
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: GAPDH, cyto, oxidative stress 24h, active, single subunit...
| File | emd_29663_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, active, single subunit analysis, class4 | ||||||||||||
| Projections & Slices |
| ||||||||||||
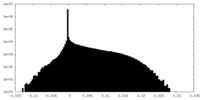
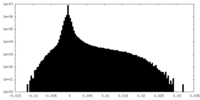
| Density Histograms |
-Additional map: GAPDH, cyto, oxidative stress 24h, inactive, single subunit...
| File | emd_29663_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, inactive, single subunit analysis, class1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
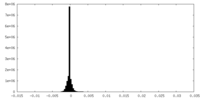
| Density Histograms |
-Additional map: GAPDH, cyto, oxidative stress 24h, nondefined, single subunit...
| File | emd_29663_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, nondefined, single subunit analysis, class2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
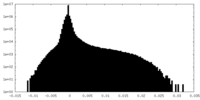
| Density Histograms |
-Additional map: GAPDH, cyto, oxidative stress 24h, nondefined, single subunit...
| File | emd_29663_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, nondefined, single subunit analysis, class3 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: GAPDH, cyto, oxidative stress 24h, nondefined, single subunit...
| File | emd_29663_additional_5.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, nondefined, single subunit analysis, class5 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: GAPDH, cyto, oxidative stress 24h, halfmap1, d2
| File | emd_29663_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, halfmap1, d2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: GAPDH, cyto, oxidative stress 24h, halfmap2, d2
| File | emd_29663_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GAPDH, cyto, oxidative stress 24h, halfmap2, d2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GAPDH
| Entire | Name: GAPDH Glyceraldehyde 3-phosphate dehydrogenase Glyceraldehyde 3-phosphate dehydrogenase |
|---|---|
| Components |
|
-Supramolecule #1: GAPDH
| Supramolecule | Name: GAPDH / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Glyceraldehyde-3-phosphate dehydrogenase
| Macromolecule | Name: Glyceraldehyde-3-phosphate dehydrogenase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO EC number:  glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 35.967969 KDa |
| Sequence | String: GKVKVGVNGF GRIGRLVTRA AFNSGKVDIV AINDPFIDLN YMVYMFQYDS THGKFHGTVK AENGKLVING NPITIFQERD PSKIKWGDA GAEYVVESTG VFTTMEKAGA HLQGGAKRVI ISAPSADAPM FVMGVNHEKY DNSLKIISNA SCTTNCLAPL A KVIHDNFG ...String: GKVKVGVNGF GRIGRLVTRA AFNSGKVDIV AINDPFIDLN YMVYMFQYDS THGKFHGTVK AENGKLVING NPITIFQERD PSKIKWGDA GAEYVVESTG VFTTMEKAGA HLQGGAKRVI ISAPSADAPM FVMGVNHEKY DNSLKIISNA SCTTNCLAPL A KVIHDNFG IVEGLMTTVH AITATQKTVD GPSGKLWRDG RGALQNIIPA STGAAKAVGK VIPELNGKLT GMAFRVPTAN VS VVDLTCR LEKPAKYDDI KKVVKQASEG PLKGILGYTE HQVVSSDFNS DTHSSTFDAG AGIALNDHFV KLISWYDNEF GYS NRVVDL MAHMASKE UniProtKB:  Glyceraldehyde-3-phosphate dehydrogenase Glyceraldehyde-3-phosphate dehydrogenase |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 45.8 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.07 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 598578 |
 Movie
Movie Controller
Controller










 Z
Z Y
Y X
X