[English] 日本語
 Yorodumi
Yorodumi- EMDB-28540: BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinemen... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
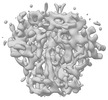
| Title | BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with a mask around the nanoparticle core | |||||||||
 Map data Map data | BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with a mask around the nanoparticle core - Main Map | |||||||||
 Sample Sample |
| |||||||||
| Biological species |    Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.7 Å cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Antanasijevic A / Zhang YN / Zhu J / Ward AB | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Single-component multilayered self-assembling protein nanoparticles presenting glycan-trimmed uncleaved prefusion optimized envelope trimmers as HIV-1 vaccine candidates. Authors: Yi-Nan Zhang / Jennifer Paynter / Aleksandar Antanasijevic / Joel D Allen / Mor Eldad / Yi-Zong Lee / Jeffrey Copps / Maddy L Newby / Linling He / Deborah Chavez / Pat Frost / Anna Goodroe / ...Authors: Yi-Nan Zhang / Jennifer Paynter / Aleksandar Antanasijevic / Joel D Allen / Mor Eldad / Yi-Zong Lee / Jeffrey Copps / Maddy L Newby / Linling He / Deborah Chavez / Pat Frost / Anna Goodroe / John Dutton / Robert Lanford / Christopher Chen / Ian A Wilson / Max Crispin / Andrew B Ward / Jiang Zhu /   Abstract: Uncleaved prefusion-optimized (UFO) design can stabilize diverse HIV-1 envelope glycoproteins (Envs). Single-component, self-assembling protein nanoparticles (1c-SApNP) can display 8 or 20 native- ...Uncleaved prefusion-optimized (UFO) design can stabilize diverse HIV-1 envelope glycoproteins (Envs). Single-component, self-assembling protein nanoparticles (1c-SApNP) can display 8 or 20 native-like Env trimers as vaccine candidates. We characterize the biophysical, structural, and antigenic properties of 1c-SApNPs that present the BG505 UFO trimer with wildtype and modified glycans. For 1c-SApNPs, glycan trimming improves recognition of the CD4 binding site without affecting broadly neutralizing antibodies (bNAbs) to major glycan epitopes. In mice, rabbits, and nonhuman primates, glycan trimming increases the frequency of vaccine responders (FVR) and steers antibody responses away from immunodominant glycan holes and glycan patches. The mechanism of vaccine-induced immunity is examined in mice. Compared with the UFO trimer, the multilayered E2p and I3-01v9 1c-SApNPs show 420 times longer retention in lymph node follicles, 20-32 times greater presentation on follicular dendritic cell dendrites, and up-to-4 times stronger germinal center reactions. These findings can inform future HIV-1 vaccine development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28540.map.gz emd_28540.map.gz | 498.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28540-v30.xml emd-28540-v30.xml emd-28540.xml emd-28540.xml | 21.1 KB 21.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28540_fsc.xml emd_28540_fsc.xml | 18.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_28540.png emd_28540.png | 191 KB | ||
| Masks |  emd_28540_msk_1.map emd_28540_msk_1.map | 536.4 MB |  Mask map Mask map | |
| Others |  emd_28540_half_map_1.map.gz emd_28540_half_map_1.map.gz emd_28540_half_map_2.map.gz emd_28540_half_map_2.map.gz | 430.9 MB 429.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28540 http://ftp.pdbj.org/pub/emdb/structures/EMD-28540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28540 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28540.map.gz / Format: CCP4 / Size: 536.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28540.map.gz / Format: CCP4 / Size: 536.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with a mask around the nanoparticle core - Main Map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_28540_msk_1.map emd_28540_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with...
| File | emd_28540_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with a mask around the nanoparticle core - Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with...
| File | emd_28540_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with a mask around the nanoparticle core - Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinemen...
| Entire | Name: BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with a mask around the nanoparticle core |
|---|---|
| Components |
|
-Supramolecule #1: BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinemen...
| Supramolecule | Name: BG505 UFO-E2p-L4P nanoparticle reconstructed by focused refinement with a mask around the nanoparticle core type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: all Details: The map was generated by focused refinement of the BG505 UFO-E2p-L4P nanoparticle dataset using a mask around the nanoparticle core (masking out the flexibly linked antigens). |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
-Macromolecule #1: BG505 UFO-E2p-L4P
| Macromolecule | Name: BG505 UFO-E2p-L4P / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 109.370852 KDa |
| Recombinant expression | Organism:   Cricetulus griseus (Chinese hamster) Cricetulus griseus (Chinese hamster) |
| Sequence | String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARSRAEN LWVTVYYGVP VWKDAETTLF CASDAKAYDT EKHNVWATHA CVPTDPNPQ EIHLENVTEE FNMWKNNMVE QMHTDIISLW DQSLKPCVKL TPLCVTLQCT NVTNNITDDM RGELKNCSFN M TTELRDKK ...String: MDAMKRGLCC VLLLCGAVFV SPSQEIHARF RRGARSRAEN LWVTVYYGVP VWKDAETTLF CASDAKAYDT EKHNVWATHA CVPTDPNPQ EIHLENVTEE FNMWKNNMVE QMHTDIISLW DQSLKPCVKL TPLCVTLQCT NVTNNITDDM RGELKNCSFN M TTELRDKK QKVYSLFYRL DVVQINENQG NRSNNSNKEY RLINCNTSAI TQACPKVSFE PIPIHYCAPA GFAILKCKDK KF NGTGPCP SVSTVQCTHG IKPVVSTQLL LNGSLAEEEV MIRSENITNN AKNILVQFNT PVQINCTRPN NNTRKSIRIG PGQ AFYATG DIIGDIRQAH CNVSKATWNE TLGKVVKQLR KHFGNNTIIR FANSSGGDLE VTTHSFNCGG EFFYCNTSGL FNST WISNT SVQGSNSTGS NDSITLPCRI KQIINMWQRI GQAMYAPPIQ GVIRCVSNIT GLILTRDGGS TNSTTETFRP GGGDM RDNW RSELYKYKVV KIEPLGVAPT RCKRRVVGGG GGSGGGGSAV GIGAVFLGFL GAAGSTMGAA SMTLTVQARN LLSGNP DWL PDMTVWGIKQ LQARVLAVER YLRDQQLLGI WGCSGKLICC TNVPWNSSWS NRNLSEIWDN MTWLQWDKEI SNYTQII YG LLEESQNQQE KNEQDLLALD ASGAAAKPAT TEGEFPETRE KMSGIRRAIA KAMVHSKHTA PHVTLMDEAD VTKLVAHR K KFKAIAAEKG IKLTFLPYVV KALVSALREY PVLNTSIDDE TEEIIQKHYY NIGIAADTDR GLLVPVIKHA DRKPIFALA QEINELAEKA RDGKLTPGEM KGASCTITNI GSAGGQWFTP VINHPEVAIL GIGRIAEKPI VRDGEIVAAP MLALSLSFDH RMIDGATAQ KALNHIKRLL SDPELLLMGG GGSFSEEQKK ALDLAFYFDR RLTPEWRRYL SQRLGLNEEQ IERWFRRKEQ Q IGWSHPQF EKGSAKFVAA WTLKAAA |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.1 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: TBS buffer prepared from a 10X stock | |||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: OTHER | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV / Details: Blotting time varied between 3 and 7 seconds.. | |||||||||
| Details | The nanoparticle was expressed in ExpiCHO cells and purified using a combination of immuno-affinity chromatography and SEC. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 29000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-41 / Number grids imaged: 1 / Number real images: 2300 / Average exposure time: 10.25 sec. / Average electron dose: 50.2 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X



























